Continuous versus intermittent infusion of β-lactams in patients with sepsis and septic shock: a systematic review and meta-analysis
- PMID: 41013406
- PMCID: PMC12465841
- DOI: 10.1186/s12879-025-11504-2
Continuous versus intermittent infusion of β-lactams in patients with sepsis and septic shock: a systematic review and meta-analysis
Abstract
Objective: To assess whether continuous infusion of β-lactam antibiotics improves clinical outcomes compared to intermittent infusion in adult patients with sepsis or septic shock.
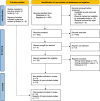
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials comparing continuous versus intermittent β-lactam infusion. Databases searched included PubMed, Scopus, Web of Science, and Embase. Risk of bias was assessed using the RoB 2.0 tool, and the certainty of evidence was evaluated using GRADE.
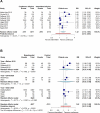
Results: Eleven studies involving 9,166 patients were analyzed, comparing continuous versus intermittent infusion of β-lactams in sepsis or septic shock. There was no significant difference in overall mortality (RR 0.94; 95% CI: 0.88-1.01) or ICU mortality (RR 0.94; 95% CI: 0.88-1.01). Continuous infusion was associated with lower hospital mortality (RR 0.92; 95% CI: 0.85-0.99), higher survival at the end of the study (RR 1.04; 95% CI: 1.02-1.07), and higher clinical cure rate (RR 1.42; 95% CI: 1.12-1.80). No significant differences were observed in the length of stay in the ICU (MD 0.75 days; 95% CI: -1.17 to 2.68) or hospital stay (MD -2.51 days; 95% CI: -10.13 to 5.12), or in the adverse events (RR 0.82; 95% CI: 0.60-1.12).
Conclusion: Continuous infusion of β-lactams could reduce hospital mortality and increase the clinical cure rate in critically ill patients, although its effect on overall mortality, hospital stay, and adverse events remains uncertain. PROSPERO number: CRD42024613938.
Keywords: Beta lactam antibiotics; Continuous infusion; Intermittent infusion; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors consent to the publication of the manuscript. Competing interests: The authors declare that they have no competing interests. Clinical trial: Not applicable.
Figures




References
-
- I M-L RF. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42. 10.1097/CCM.0000000000000330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

