Injectable responsive hydrogel with synergistic antibacterial and anti-inflammatory properties for enhanced periodontitis treatment
- PMID: 41013665
- PMCID: PMC12465922
- DOI: 10.1186/s12951-025-03698-z
Injectable responsive hydrogel with synergistic antibacterial and anti-inflammatory properties for enhanced periodontitis treatment
Abstract
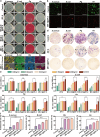
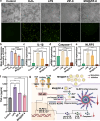
Periodontitis is a chronic inflammatory disease driven by dysbiotic microbial biofilms and localized reactive oxygen species (ROS) accumulation, with inflammation management made challenging by recurrent infections from residual pathogenic bacteria in the periodontal pockets. To address this, we engineered an injectable pH-responsive hydrogel (MH@ZIF-8/CS/β-GP) through the integration of minocycline hydrochloride (MH)-encapsulated zeolitic imidazolate framework-8 (ZIF-8) nanoparticles into a chitosan (CS) and β-glycerophosphate (β-GP) crosslinked matrix. The MH@ZIF-8 displayed broad-spectrum antimicrobial efficacy against key periodontal pathogens including Porphyromonas gingivalis (Pg), and Aggregatibacter actinomycetemcomitans (Aa), primarily attributed to the synergistic antimicrobial effects of Zn ions and MH. Additionally, MH@ZIF-8 effectively eliminated ROS by inhibiting the NLRP3/Caspase-1/IL-1β signaling pathway, demonstrating potent anti-inflammatory effect in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. The MH@ZIF-8/CS/β-GP hydrogel, which exhibited favored cytocompatibility with human gingival fibroblasts (HGFs), undergoed a rapid sol-gel transition and pH-responsive sustained-release drug delivery under the acidic conditions of periodontal pockets, effectively responding to periodontitis microenvironment. Meanwhile, this hydrogel effectively alleviated alveolar bone loss in vivo. Overall, the developed MH@ZIF-8/CS/β-GP hydrogel presents a novel strategy for chronic periodontitis treatment and demonstrates promising clinical application potential.
Keywords: Anti-bacteria; Anti-inflammation; Chronic periodontitis; Responsive hydrogel.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the Ethics Committee of the School of Stomatology, Lanzhou University (No. LZUKQ-2023-060). Consent for publication: All authors agree for publication. Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
- 82370926/National Natural Science Foundation of China
- 25YFFA026/Gansu Provincial Key Research and Development Program
- 071100255/Gansu Province Key Laboratory of Craniofacial Reconstruction and Bio-intelligent Manufacturing Open Research Topics
- lzujbky-2025-24/Fundamental Research Funds for the Central Universities
LinkOut - more resources
Full Text Sources
Medical

