Biomimetic Cu2-xSe nanoplatforms for efficient glioblastoma treatment: overcoming the blood-brain barrier and boosting Immunogenetic cell death
- PMID: 41013700
- PMCID: PMC12465653
- DOI: 10.1186/s12951-025-03696-1
Biomimetic Cu2-xSe nanoplatforms for efficient glioblastoma treatment: overcoming the blood-brain barrier and boosting Immunogenetic cell death
Abstract
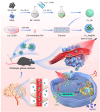
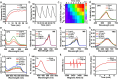
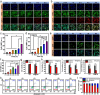
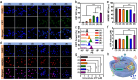
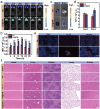
Glioblastoma (GBM) is an aggressive and highly heterogeneous brain tumor that continues to pose a significant clinical challenge. Current therapeutic strategies, including surgical resection, radiotherapy, and chemotherapy, are hindered by the tumor's invasive behavior, resistance to treatment, and the difficulty of selectively targeting tumor cells. Emerging modalities, such as immunotherapy and photodynamic therapy, hold considerable promise; however, their efficacy in treating GBM is limited by critical barriers, including poor penetration of the blood-brain barrier (BBB), tumor heterogeneity, and insufficient accumulation of therapeutic agents at the tumor site. In this study, innovative biomimetic copper selenide nanoparticles (CS@CM) are developed for targeted photothermal therapy of GBM. These nanoparticles are functionalized with glioma cell membranes (CM), and this biomimetic design leverages the homing capability of the membranes to achieve efficient BBB penetration and enhanced targeting of GBM tissues. CS@CM act as potent photothermal agents upon light activation, which can amplify reactive oxygen species-induced oxidative stress to damage glioma cells. Such combination therapy effectively triggers immunogenic cell death to achieve splendid antitumor efficacy, offering a promising therapeutic strategy for GBM. Collectively, this approach addresses the limitations of conventional treatments, paving the way for improved clinical outcomes in managing this formidable malignancy.
Keywords: Blood-brain barrier penetration; Glioblastoma therapy; Immunogenetic cell death; Oxidative damage.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- KJQN202100467/Scientific and Technological Research Program of Chongqing Municipal Education Commission
- KJQN202400202/Scientific and Technological Research Program of Chongqing Municipal Education Commission
- 2024YFFK0249/Key Research and Development Project of Sichuan Provincial Science and Technology Plan
- KFKT202405/Open Research Project from Anhui Provincial Key Laboratory of Tumor Evolution and Intelligent Diagnosis and Treatment
LinkOut - more resources
Full Text Sources
Medical

