Combination immunotherapy in hepatocellular carcinoma: synergies among immune checkpoints, TKIs, and chemotherapy
- PMID: 41013723
- PMCID: PMC12465164
- DOI: 10.1186/s13045-025-01739-6
Combination immunotherapy in hepatocellular carcinoma: synergies among immune checkpoints, TKIs, and chemotherapy
Abstract
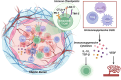
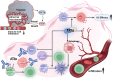
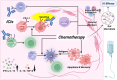
Combination therapy is rapidly becoming the cornerstone of hepatocellular carcinoma (HCC) treatment. Immune checkpoint inhibitors (ICIs) have emerged as a central strategy in systemic therapy, yet their efficacy as monotherapies remains limited. Consequently, combinatorial approaches, such as ICIs-Tyrosine kinase inhibitors (TKIs), ICIs-chemotherapy, and dual ICI regimens, are gaining momentum. While clinical trials have established efficacy benchmarks, mechanistic insights remain scarce, partly due to the limitations of current preclinical models in mimicking the complex tumor microenvironment (TME). Given the substantial heterogeneity of HCC, spanning genetic, transcriptomic, and immunologic dimensions, treatment outcomes vary widely. Additional factors such as gut microbiota and epigenetic modifications further influence therapeutic response and resistance. Although PD-1, PD-L1, and CTLA-4 inhibitors are widely used, unresponsiveness is common. Novel targets such as LAG-3, TIM-3, TIGIT, and VISTA, as well as strategies to reprogram fibrotic and immunosuppressive TME, are under active investigation. Ultimately, translating basic insights into personalized therapy will depend on predictive biomarkers and integrated analyses that account for the complex interactions among tumor cells, the immune system, and the TME. This review synthesizes current knowledge and cellular mechanisms underpinning combination therapies, highlights therapeutic synergies, and discusses emerging directions for stratified treatment in HCC.
Keywords: Chemotherapy; Combination immunotherapy; Hepatocellular carcinoma (HCC); Immune checkpoint inhibitors (ICIs); Tyrosine kinase inhibitors (TKIs).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–62. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. - PubMed
-
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

