Effect of controlled human Plasmodium falciparum infection on B cell subsets in individuals with different levels of malaria immunity
- PMID: 41014333
- PMCID: PMC12476306
- DOI: 10.1007/s00430-025-00847-x
Effect of controlled human Plasmodium falciparum infection on B cell subsets in individuals with different levels of malaria immunity
Abstract
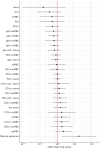
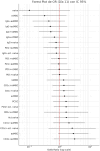
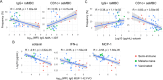
Continuous exposure to Plasmodium falciparum (Pf) has been associated with alterations in B cells. We investigated the effect of controlled human malaria infection (CHMI) on B cell phenotypes in individuals with different Pf immunity status: malaria-naïve, immunized with PfSPZ-CVac and semi-immune (lifelong-exposed) volunteers. Compared to naïve, semi-immune but not vaccinated individuals, had increased baseline frequencies of immature B cells (CD19+CD10+), active naive (IgD+CD27-CD21-) B cells, active atypical (IgD-CD27-CD21-) memory B cells (MBCs), active classical (IgD-CD27+CD21-) MBCs and CD1c+-B cells but lower frequencies of some IgG+-B cells. The frequencies of CD1c+ active atypical MBCs correlated positively with anti-Pf antibodies and negatively with circulating eotaxin levels, while the opposite was observed for IgG+ resting atypical MBCs. During early blood-stage infection (day 11 after CHMI), there was an expansion of resting classical (IgD-CD27+CD21+) MBCs in all three groups. Vaccination, compared to placebo, altered the effect of CHMI on B cells, showing a positive association with resting classical MBCs (β = 0.190, 95% CI 0.011-0.368) and active naïve-PD1+ (β = 0.637, 95% CI 0.058 to 1.217) frequencies, and a negative one with CD1c+ resting atypical MBCs (β = - 0.328, 95% CI - 0.621 to - 0.032). In addition, the sickle cell trait in semi-immune subjects altered the effect of CHMI on several B cells. In conclusion, lifelong but not vaccine exposure to malaria was associated with increased frequencies of multiple B cell subsets, with higher and lower percentages of CD1c and IgG expressing-cells, respectively. A single infection (CHMI) induces changes in B cell frequencies and is modulated by sickle cell trait and malaria-immunity status.Clinical Trials Registration NCT01624961, NCT02115516, and NCT02237586.
Keywords: Plasmodium falciparum; B cells; Controlled human malaria infection; Cytokines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: E.R.J., B.K.L.S., and S.L.H. work for Sanaria Inc., a company that produces the Plasmodium falciparum sporozoite products used in the studies described in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Figures





Update of
-
Effect of controlled human Plasmodium falciparum infection on B cell subsets in individuals with different levels of malaria immunity.Res Sq [Preprint]. 2025 Apr 19:rs.3.rs-6221433. doi: 10.21203/rs.3.rs-6221433/v1. Res Sq. 2025. Update in: Med Microbiol Immunol. 2025 Sep 27;214(1):47. doi: 10.1007/s00430-025-00847-x. PMID: 40321757 Free PMC article. Updated. Preprint.
References
-
- Geneva: World Health Organization. World malaria report 2024: addressing inequity in the global malaria response. [Internet]. Available from: https://www.wipo.int/amc/en/mediation/https://www.who.int/teams/global-malaria-programme/reports/world-malaria... (2024).
-
- RTS S Clinical Trials Partnership (2015) Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. The Lancet 386:31–45 - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

