Once-daily dolutegravir/lamivudine fixed-dose formulations in children living with HIV: a pharmacokinetic and safety sub-study nested in the open-label, multicentre, randomised, non-inferiority D3/PENTA 21 trial
- PMID: 41014973
- PMCID: PMC12509724
- DOI: 10.1016/j.ebiom.2025.105929
Once-daily dolutegravir/lamivudine fixed-dose formulations in children living with HIV: a pharmacokinetic and safety sub-study nested in the open-label, multicentre, randomised, non-inferiority D3/PENTA 21 trial
Abstract
Background: Two-drug regimen dolutegravir/lamivudine (DTG/3TC) is recommended as an alternative to standard three-drug regimens in adult treatment guidelines. This nested pharmacokinetic sub-study within the D3/Penta 21 randomised trial (#NCT04337450) assessed DTG and 3TC concentrations and safety in virologically-suppressed children, switching to once-daily DTG/3TC fixed-dose formulations.
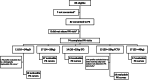
Methods: Children aged 2-<15 years received either 5/30 mg DTG/3TC dispersible tablets (DT) or 50/300 mg film-coated tablets (FCT), using WHO weight band (WB)-aligned dosing: 10-<14 kg 4 DTs; 14-<20 kg 5 DTs; 20-<25 kg 6 DTs or 1 FCT; 25-<40 kg 1 FCT. A minimum of 8-evaluable pharmacokinetic curves per WB/formulation were targeted for 24 h pharmacokinetic profiling (t = 0, 1, 2, 3, 4, 6, and 24 h post-dosing) at steady state. The number of children with DTG Ctrough <0.32 mg/L (EC90) and <0.064 mg/L (PA-IC90) were summarised. Safety was evaluated through 48 weeks in eligible children consented to the pharmacokinetic sub-study.
Findings: Between 11th May 2022 and 31st May 2023, 82 children consented for the sub-study. Seventy-two were included in the pharmacokinetic analysis; median (IQR) age was 7.1 (4.9-10.0) years and weight 21.6 (17.7-24.8) kg. DTG geometric mean (GM) (%CV) Ctrough and AUC0-24 h were 0.82 (54) mg/L and 66.2 (35) h∗mg/L. 3TC GM-AUC0-24 h was 16.2 (45) h∗mg/L. Three children had DTG Ctrough<0.32 mg/L, all had DTG Ctrough ≥0.064 mg/L. In children weighing 20-<25 kg WB and taking 1 FCT (50/300 mg) 3TC GM AUC0-24 h was 19% higher than in children ≥25 kg (1 FCT). Of 82 children, 3 had 4 serious adverse events (SAEs) and 5 had 6 grade ≥3 adverse events (AEs). No AEs were related to DTG/3TC or resulted in treatment discontinuation. No 3TC-related AEs or laboratory abnormalities were observed in children taking FCT in the 20-<25 kg WB. PK parameters were comparable to historical paediatric data from ODYSSEY (DTG) and IMPAACT2019 (3TC) trials.
Interpretation: The study demonstrated adequate DTG and 3TC exposures with reassuring safety profiles using WB-based dosing, supporting licencing applications for dispersible and film-coated DTG/3TC formulations for paediatric use.
Funding: The D3/Penta 21 trial is sponsored by Fondazione Penta Onlus ETS (Penta) and funded by ViiV Healthcare.
Keywords: Dolutegravir; Fixed-dose formulation; HIV; Lamivudine; Paediatric; Two-drug regimen.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Ann M. Buchanan and Justine Boles are employees of ViiV Healthcare and receives stock options from GSK. Isabelle Deprez is an employee of Certara, owns Certara shares, and is also a complementary worker for GSK. Anna Turkova and Man K. Chan receives core support from the UK Medical Research Council (grants MC_UU_00004/03). Avy Violari received funding for the parent study (institutional payments). Angela Colbers received grants from Gilead, ViiV, and Merck (via Radboudumc), and consulting fees from Gilead. David Burger received funding from ViiV for the PANNA study and a ViiV-sponsored lecture (both paid to institution). Moherndran Archary holds an NIHR Global Research Professorship. Justine Boles holds GSK shares. Pauline Amuge reports study support from PENTA Fondazione at Baylor-Uganda. Saskia de Wildt received institutional funding from EU IMI2, Roche, and the Gates Foundation. She is co-inventor on a pending patent (institution to receive any proceeds) and serves on advisory boards and in leadership roles for various academic and nonprofit organisations, mostly unpaid or institutionally compensated.
Figures


References
-
- UNAIDS . 2024. Global aids update.
-
- WHO . 2021. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring. - PubMed
-
- Clinton Health Access Initiative IC HIV market report: the state of HIV treatment, testing, and prevention in low- and middle- income countries. 2024. 2024-HIV-Market-Report_12.20.24.pdf 14. Available from:
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

