Quantitative Time-Dependent Diffusion MRI for Diagnosis and Aggressiveness Assessment of Endometrial Cancer: A Prospective Study
- PMID: 41015861
- PMCID: PMC12479230
- DOI: 10.3348/kjr.2025.0633
Quantitative Time-Dependent Diffusion MRI for Diagnosis and Aggressiveness Assessment of Endometrial Cancer: A Prospective Study
Abstract
Objective: Preoperative differentiation of benign and malignant endometrial lesions, along with the identification of aggressive histological types of endometrial cancer (EC), is crucial for guiding treatment strategies. Time-dependent diffusion magnetic resonance imaging (TDD-MRI), which allows the characterization of tissue microstructure at the cellular level, is not currently applied for endometrial lesions. This study aimed to evaluate TDD-MRI-derived microstructural parameters for noninvasively distinguishing benign and malignant endometrial lesions and predicting aggressive histological types of EC.
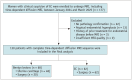
Materials and methods: This prospective study enrolled 177 patients with clinically suspected EC who underwent TDD-MRI between January 2024 and March 2025. The Imaging Microstructural Parameters Using Limited Spectrally Edited Diffusion method was used to extract microstructural parameters, including the cell diameter (d), intracellular volume fraction (vin), cellularity (number of cells per unit area), cellularity index (vin/d), and extracellular diffusivity (Dex), along with three apparent diffusion coefficient measurements. The area under the receiver operating characteristic curve (AUC) was used to assess diagnostic performance. The Pearson correlation coefficient between the microstructural parameters and histopathological measurements was calculated.
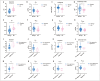
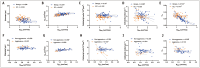
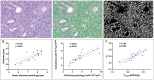
Results: A total of 130 women (mean ± standard deviation age: 56 ± 14 years) administered uterine curettage or surgery were included in the final analysis. All microstructural parameters showed significant differences between benign endometrial lesions and EC (P < 0.05), as well as between nonaggressive and aggressive EC (P < 0.05). Cellularity exhibited the highest AUC of 0.86 for distinguishing benign endometrial lesions from EC, whereas the cellularity index showed the highest AUC of 0.88 for distinguishing aggressive histological types. D0Hz was positively correlated with Dex (P < 0.05) and negatively correlated with diameter (P < 0.05), cellularity index (P < 0.01) and vin (P < 0.001) in patients with benign endometrial lesions. D0Hz was positively correlated with Dex (P < 0.001) and negatively correlated with vin (P < 0.001) in patients with EC. Microstructural parameters strongly correlated with corresponding pathological features (r = 0.77-0.83; P < 0.001).
Conclusion: TDD-MRI-derived microstructural parameters demonstrated high performance in differentiating benign from malignant endometrial diseases and identifying aggressive types of EC.
Keywords: Endometrial cancer; Histological types; Magnetic resonance imaging; Microstructural parameters; Time-dependent diffusion MRI.
Copyright © 2025 The Korean Society of Radiology.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures



References
-
- Lefebvre TL, Ueno Y, Dohan A, Chatterjee A, Vallières M, Winter-Reinhold E, et al. Development and validation of multiparametric MRI-based radiomics models for preoperative risk stratification of endometrial cancer. Radiology. 2022;305:375–386. - PubMed
MeSH terms
Grants and funding
- DFL20220303/Beijing Hospitals Authority's Ascent Plan/China
- Beijing Key Specialists in Major Epidemic Prevention and Control/China
- Beijing Science and Technology Cross-disciplinary New Star Project/China
- 62476180/NNSFC/National Natural Science Foundation of China/China
- 62176267/NNSFC/National Natural Science Foundation of China/China
LinkOut - more resources
Full Text Sources

