Pharmacomicrobiomics
- PMID: 41017122
- PMCID: PMC12641081
- DOI: 10.1002/cpt.70066
Pharmacomicrobiomics
Abstract
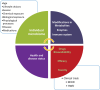
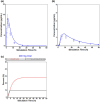
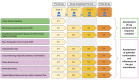
Oral medications encounter gut commensal microbes that participate directly and indirectly in drug effects through metabolism, interactions with drug metabolites, or production of substrates that compete with drugs for drug-metabolizing enzymes, consequently influencing drug pharmacokinetics. The microbiota can also affect drug efficacy or toxicity by modulating the immune system; for example, variability in response to cancer immunotherapy, such as anti-PD-1 and anti-CTLA-4 therapies, is increasingly attributed to differences in gut microbial composition and function. These conditions indicate the need and opportunity to intentionally leverage the microbiome for drug effect; as such, the study of how intra- and inter-individual differences in the microbiome affect drug response has gained a definition termed pharmacomicrobiomics. While the need is clear, clinical studies evaluating pharmacomicrobiomic interactions are challenging due to microbiome variability, multiple potential confounders, no standardization of statistical and bioinformatics methods, and the reluctance of potential clinical study participants. In this review, we make the case for pharmacomicrobiomic clinical studies; for the use of modeling and simulation to provide a quantitative framework for data integration, hypothesis testing, and translational-to-late-stage clinical predictions; and the application of real-world data to support both using a within-subject comparison approach. We argue that an integrated and cohesive approach can address the large "inherent" inter-individual variability in the microbiome, attributed to factors such as age, lifestyle choices, environmental factors, chemical and biological exposures, and disease. In summary, there are many challenges to pharmacomicrobiomics research but also enormous potential to improve the development and utilization of pharmaceutical products.
© 2025 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
References
-
- Gutierrez Lopez, D.E. , Lashinger, L.M. , Weinstock, G.M. & Bray, M.S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 33, 873–887 (2021). - PubMed
-
- Joos, R. et al. Examining the healthy human microbiome concept. Nat. Rev. Microbiol. 23, 192–205 (2024). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

