Pathways to translation for nanomedicine in nephrology
- PMID: 41018277
- PMCID: PMC12461149
- DOI: 10.1093/ckj/sfaf192
Pathways to translation for nanomedicine in nephrology
Abstract
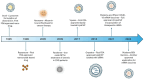
Kidney diseases are a substantial worldwide health burden, with high mortality and increasing incidence. Despite their prevalence, substantial gaps remain in the clinic in both diagnostics and therapeutics. Many novel treatments have failed in clinical trials or fallen out of use in the clinic due to side effects and poor efficacy, in large part due to poor therapeutic profiles in the kidney. Nanomedicines have begun to emerge as a potentially promising diagnostic or therapeutic delivery system. Based on their physicochemical properties, such as size, shape, surface chemistry, and so on, some nanotechnologies can target the kidneys. However, as of yet, no kidney-specific nanomedicines have reached clinical translation. While the field of renal nanomedicine is in its early stages and growing, some potential obstacles to translation include poor preclinical models, challenges in manufacturing scale-up, clinical trial design and the cost of translation. Here, we overview the current state of the kidney-targeting nanomedicine field and outline a potential framework for clinical translation. We focus on the paths of US Food and Drug Administration- approved nanomedicines and suggestions from other nanomedicine fields to inform our key considerations for translational success. We also highlight the importance of academic and clinical collaboration with industry and federal regulators. Several investigational technologies are just now at the cusp of scaling towards the clinic and we therefore aim to support this momentum for improving the lives of patients with kidney diseases.
Keywords: drug delivery; imaging; kidney targeting; nanomedicine; preclinical development.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
R.M.W. is the founder of Zipcode Therapeutics.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

