Velocity Map Imaging of NO (A) from Photodissociation of the Ar-NO Complex
- PMID: 41018567
- PMCID: PMC12461358
- DOI: 10.1021/acsomega.5c06670
Velocity Map Imaging of NO (A) from Photodissociation of the Ar-NO Complex
Abstract
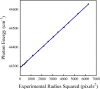
We have photodissociated the Ar-NO complex from 4 to 337 cm-1 above the energy required to form NO (A 2∑+). In the experiment, the NO (A) product was nonresonantly ionized, and velocity map ion images were recorded at a resolution sufficient to identify individual rotational states. From the data, we determined the minimum energy required to form the NO (A) product to be 44294.3 ± 2.2 cm-1. We also determined the ground state dissociation energy for Ar-NO to be 95.4 ± 2.2 cm-1 and the excited-state dissociation energy to be 52 ± 2 cm1. The ground-state dissociation energy agrees with experimental results and a recent three-dimensional ab initio potential energy surface; however, the excited-state dissociation energy is larger than predicted by theoretical surfaces. We also determined the NO (A) rotational state distribution, which was bimodal due to the rotational rainbow effect and showed significantly less contribution from hotbands compared with previous experiments. Our data also allowed for measurement of the angular anisotropy parameter, β, over the range of NO (A) rotational states. We observed that for the lowest rotational levels, β changes from ∼ -0.3 at low excitation energy to ∼+0.4 at high excitation energy, which is consistent with previous work. Furthermore, we report β values for the highest rotational states formed at each excitation energy. Our experiment demonstrated that for a given excitation energy, the high rotational states consistently exhibited a more negative β compared with the low rotational levels. Qualitatively, this behavior can be attributed to excitation to regions of the excited state resembling a skewed T-shape that then dissociate to high NO (A) rotational levels with β ≤ 0.
© 2025 The Authors. Published by American Chemical Society.
Figures




References
-
- Huber, K. P. ; Herzberg, G. H. . Constants of Diatomic Molecules NIST Chemistry WebBook, NIST Standard Reference Database Number 69. NIST Chemistry WebBook; Linstrom, P. J. ; Mallard, W. G. ;National Institute Of Standards And Technology: Gaithersburg MD, 20899
-
- Schmelz T., Rosmus P., Alexander M. H.. Theoretical Study of Bound States of Ar-NO. J. Phys. Chem. 1994;98:1073–1079. doi: 10.1021/j100055a006. - DOI
-
- Kim Y., Fleniken J., Meyer H., Alexander M. H., Dagdigian P. J.. A joint theoretical–experimental investigation of the lower bound states of the NO (X 2Π)–Ar complex. J. Chem. Phys. 2000;113:73–85. doi: 10.1063/1.481776. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
