Hyaluronic Acid Immersion Enhances Gamma-Ray-Irradiation Cross-Linking of the Fish-Derived Type I Collagen Membrane
- PMID: 41018581
- PMCID: PMC12461391
- DOI: 10.1021/acsomega.5c07582
Hyaluronic Acid Immersion Enhances Gamma-Ray-Irradiation Cross-Linking of the Fish-Derived Type I Collagen Membrane
Abstract
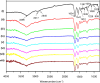
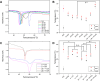
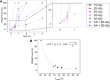
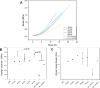
Fish-derived type I collagen is widely used in biomedical implants; however, its limited thermal and enzymatic stability necessitates cross-linking for clinical applications. This study investigated gamma-ray-induced cross-linking of collagen as a shape of membranes, with enhancement via polysaccharide-assisted immersion. Hydrated collagen membranes were irradiated at doses ranging from 15 kDa to 35 kGy to assess their dose-dependent effects. Irradiation at 15 kGy enhanced thermal and enzymatic stability, which declined at higher doses. To further enhance stability, the membranes were immersed in phosphate-buffered saline containing chondroitin sulfate (CS) or hyaluronic acid (HA) during irradiation at 25 kGy. HA-treated membranes showed a synergistic enhancement in thermal stability (T onset > 50 °C, T peak ∼ 54 °C) and enzymatic resistance, surpassing CS-treated and nonimmersed controls. MALDI-TOF mass spectrometry revealed the selective loss of irradiation-sensitive amino acid residues, indicating involvement in cross-linking and scission mechanisms. Tensile testing confirmed that the HA-treated membranes retained a tensile strength (∼1.7 MPa) under hydrated conditions, comparable to the irradiated-only controls. These findings demonstrate that HA-assisted gamma-irradiation at 25 kGy effectively enhances collagen cross-linking, improving its thermal and enzymatic stability while preserving its mechanical performance for biomedical applications.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
-
- Gomez-Guillen M. C., Gimenez B., Lopez-Caballero M. E., Montero M. P.. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011;25(8):1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. - DOI
LinkOut - more resources
Full Text Sources
