Outstanding Ultra-Low Freezing Tolerance in Moss Species: Insights From Recovery Ability
- PMID: 41018679
- PMCID: PMC12460189
- DOI: 10.1002/pei3.70081
Outstanding Ultra-Low Freezing Tolerance in Moss Species: Insights From Recovery Ability
Abstract
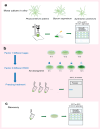
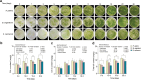
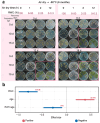
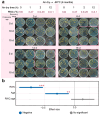
Freezing temperature is a key environmental factor that influences plant growth and distribution. Mosses exhibit remarkable resistance to freezing stress due to their unique morphological and physiological traits. The protonema, which is the initial structure formed during the germination of a moss spore, exhibits a short life cycle and is highly sensitive to environmental changes. In this study, the protonemas of three moss species, Physcomitrium patens, Bryum argenteum, and Syntrichia caninervis, were harvested when they were 5, 10, and 15 days old. Protonemas were air dried for 0, 1, 2, and 12 h. Air-dried protonemas were kept at -80°C for 6 months to evaluate their resilience to ultra-low freezing stress. This resilience was assessed at 6, 12, and 18 days after re-culture. The three moss species exhibited varying degrees of freezing tolerance. P. patens did not recover after -80°C treatment, fully dried 10-days-old B. argenteum achieved highest recovery rate of 99.6% ± 0.2% while fully dried 5-days-old S. caninervis achieved the highest recovery rate of 98.6% ± 0.5%. The regeneration rate was influenced by both relative water content (RWC) and age. An analysis using a linear mixed-effects model indicated that the impact of RWC (effect size = 0.75) was greater than that of age (effect size = 0.35). This research provides valuable insights into the resilience of moss protonemas after exposure to -80°C, emphasizing the importance of protonema in abiotic stress research. These findings are crucial for developing methods to preserve and maintain terrestrial ecosystems in arid regions.
Keywords: age; freezing stress; moss; protonema; recovery rate; relative water content.
© 2025 The Author(s). Plant‐Environment Interactions published by New Phytologist Foundation and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
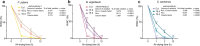

References
-
- Bai, W. W. , Salih H., Yang R. R., et al. 2024. “ScDREBA5 Enhances Cold Tolerance by Regulating Photosynthetic and Antioxidant Genes in the Desert Moss <styled-content style="fixed-case"> Syntrichia caninervis </styled-content> .” Plant, Cell and Environment 48: 3293–3313. 10.1111/pce.15336. - DOI - PubMed
-
- Bewley, J. D. 1973. “Polyribosome Extraction RNA Synthesis.” Plant Science Letters 1: 303–308. 10.1016/0304-4211(73)90093-X. - DOI
LinkOut - more resources
Full Text Sources
