Seroprevalence of antibodies to Plasmodium falciparum transmission-blocking target proteins Pfs230D1M and Pfs48/45 in Tanzanian populations of diverse malaria transmission intensity
- PMID: 41019048
- PMCID: PMC12460233
- DOI: 10.3389/fimmu.2025.1589061
Seroprevalence of antibodies to Plasmodium falciparum transmission-blocking target proteins Pfs230D1M and Pfs48/45 in Tanzanian populations of diverse malaria transmission intensity
Abstract
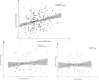
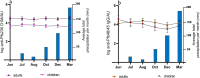
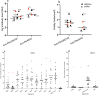
Transmission-blocking vaccines are among the novel tools under development for malaria control and elimination. Understanding the human immune response to the sexual stages of Plasmodium falciparum is essential for progressing transmission-blocking vaccine development. A serosurvey was conducted in Tanzania, from May to August 2022 among 290 participants, consisting of 114 children (5-12 years), 44 adolescents (13-17 years), and 132 adults (18-45 years). The participants were tested for malaria parasites using quantitative polymerase chain reaction, and standardized enzyme-linked immunosorbent assays were performed to detect the presence of IgG antibodies against transmission-blocking target antigens-Pfs230D1M, Pfs48/45, and Pfs25. A set of 10 plasma samples that were reactive to Pfs230DIM and/or Pfs48/45 were tested individually for transmission-reducing activity via standard membrane feeding assays. Of the participants tested, 56% (157/281) had detectable Pfs230D1M antibodies, and 49% (141/290) were positive for Pfs48/45 IgG. Approximately 30% were seropositive for both. However, Pfs25 IgG was not detected in any of the 117 participants tested. The seroprevalence for Pfs230D1M and Pfs48/45 IgG increased significantly with participants' age, with adults more likely to have antibodies than children: Pfs230D1M (adjusted odds ratio: 3.16, 95% confidence interval: 1.81-5.53, p-value ≤ 0.0001) and Pfs45/48 (OR: 3.06, 95% CI: 1.79-5.25, p ≤ 0.0001). There was no significant difference in antibody titers for Pfs230D1M and Pfs48/45 antibodies across age groups. A significant transmission-reducing activity was observed in 2/10 participants, who were highly reactive to Pfs230D1M and Pfs48/45. Naturally acquired antibody responses to both full-length Pfs48/45 and Pfs230D1M proteins are prevalent and appeared to be stable, suggesting that semi-immune populations may be ideal to evaluate boosting transmission-blocking vaccine candidates.
Keywords: Pfs230D1M; Pfs25; Plasmodium falciparum; Tanzania; and Pfs48/45; gametocytes; malaria transmission; transmission-blocking vaccines.
Copyright © 2025 Mulamba, Kalinga, Mtaka, Lazaro, Kamage, Nkumama, Odufuwa, Kreppel, Mekhaiel, Miura, Long, Olotu and Williams.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO . World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization (WHO). (2020).
-
- WHO . World malaria report 2023. Geneva: World Health Organization (Series Editor) (2023).
-
- WHO . World malaria report 2022. Geneva: World Health Organization; (2022).
-
- WHO . World malaria report 2021. Geneva: World Health Organization (Series Editor) (2021).
-
- WHO . (2024).World malaria report 2024. Geneva: World Health Organization.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

