Brexpiprazole for agitation in alzheimer's disease: A systematic review and meta-analysis of randomized controlled trials
- PMID: 41019272
- PMCID: PMC12468828
- DOI: 10.4103/indianjpsychiatry_197_25
Brexpiprazole for agitation in alzheimer's disease: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Agitation in Alzheimer's disease (AD) severely affects patients and caregivers. Brexpiprazole, a serotonin-dopamine modulator, is the potential treatment; however, recent trials and variations in dosing have raised questions about its optimal efficacy and safety.
Aim: To evaluate the efficacy and safety of brexpiprazole in the treatment of agitation associated with AD, with a focus on dose-specific outcomes.
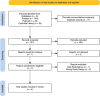
Methods: A systematic search was conducted in PubMed, Embase, and the Cochrane Library for Randomized Controlled Trials (RCT) comparing brexpiprazole with placebo in AD-related agitation. Primary efficacy outcomes included changes in the Cohen-Mansfield Agitation Inventory (CMAI) and Clinical Global Impression-Severity (CGI-S) scores. Safety outcomes encompassed treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), and mortality. Meta-analyses were performed using a random-effects model, with mean differences (MD) and odds ratios (OR) reported with 95% confidence intervals (CI).
Results: Four RCTs with 1,710 participants were included. Brexpiprazole 2 mg significantly reduced CMAI scores (MD: -5.618; 95% CI: -7.884, -3.351; P < 0.001) and CGI-S scores (MD: -0.513; 95% CI: -0.890, -0.135; P = 0.008) compared to placebo. Lower doses (0.5-1 mg) demonstrated limited efficacy. TEAEs were more frequent with brexpiprazole 2 mg (OR: 1.554; 95% CI: 1.045, 2.312; P = 0.030), while SAEs (OR: 1.389; P = 0.384) and mortality (OR: 2.189; P = 0.301) did not significantly differ from placebo.
Conclusion: Brexpiprazole 2 mg is effective in reducing agitation symptoms in AD with an acceptable safety profile.
Keywords: Agitation; Alzheimer’s disease; brexpiprazole; meta-analysis; randomized controlled trials.
Copyright: © 2025 Indian Journal of Psychiatry.
Conflict of interest statement
There are no conflicts of interest.
Figures








References
-
- Kamatham PT, Shukla R, Khatri DK, Vora LK. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res Rev. 2024;101:102481. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
