Preclinical and clinical evaluation of ECM bioenvelopes for preventing CIED pocket complications
- PMID: 41019442
- PMCID: PMC12464048
- DOI: 10.3389/fcvm.2025.1638929
Preclinical and clinical evaluation of ECM bioenvelopes for preventing CIED pocket complications
Abstract
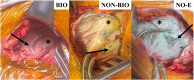
Cardiac implantable electronic device (CIED) envelopes were developed to secure the device within the surgical pocket, mitigating serious risks for migration or erosion. Available CIED envelopes are either biologic, constructed from non-crosslinked extracellular matrix (ECM), or non-biologic, composed of absorbable synthetic mesh impregnated with antibiotics. Multiple studies have documented constructive remodeling following implantation of the ECM-based bioenvelopes, leading to healthy wound healing and a vascularized surgical pocket. Non-biologic materials, in contrast, trigger a foreign-body response, leading to fibrous encapsulation of the device. Indeed, clinical studies of the bioenvelope have demonstrated constructive remodeling and integration into host tissues. One observational clinical study evaluating CIED reoperations found that patients previously implanted with the bioenvelope had well-vascularized surgical pockets with site-appropriate tissues that facilitated easier device replacement, as opposed to fibrotic encapsulation of the device in patients managed with non-biologic envelopes or without envelopes. A novel, recently approved antibiotic-eluting bioenvelope is designed to provide both support for healthy wound healing plus reduced infection risk, which is a common adverse outcome of CIED implantation. This next-generation bioenvelope includes absorbable discs impregnated with the broad-spectrum antibiotics rifampin and minocycline. Preclinical studies report excellent biocompatibility, biphasic release of antibiotics over 2 weeks, and complete eradication of bacterial inoculates commonly associated with CIED infections. Therefore, this new antibiotic eluting bioenvelope adds standardized drug delivery to the device, without compromising the wound-healing benefits of non-crosslinked ECM.
Keywords: CIED; bioenvelope; biologic envelope; extracellular matrix; minocycline; pocket infection; reoperation; rifampin.
© 2025 Catanzaro, Christopher, Issa, Nekkanti, Phan, Sangosanya, Yarmohammadi and D’Souza.
Conflict of interest statement
JC and BD receive honoraria from Elutia, Inc. The authors declare that this study received funding from Elutia Inc. The funder had the following involvement in the study: independent data collection, analysis, writing and decision for publication.
Figures
References
-
- Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythm Electrophysiol. (2016) 9:e003929. 10.1161/CIRCEP.116.003929 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

