Clinical effectiveness of HPV vaccine by age at vaccination: a matched case-control study
- PMID: 41019517
- PMCID: PMC12465035
- DOI: 10.1016/j.lana.2025.101225
Clinical effectiveness of HPV vaccine by age at vaccination: a matched case-control study
Abstract
Background: Important questions remain about the extent to which human papillomavirus (HPV) vaccines are realizing their full potential in real-world settings. This study aimed to assess how age at the time of vaccination influences its effectiveness against HPV-attributable high-grade cervical lesions (HGCL).
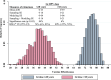
Methods: In this matched case-control study conducted in New Haven County, Connecticut, cases were vaccine-eligible women diagnosed with HGCL associated with HPV 16 or 18 from 1/1/2010 to 12/31/2019. Controls were women with normal Pap smear results, matched to cases by age, medical practice, and date of Pap test. Participants were interviewed and records were reviewed to ascertain vaccination history and possible confounders including sexual behaviors. Vaccine effectiveness (VE) by age at the time of vaccination was assessed using matched odds ratios (OR) and 95% confidence intervals (CI) derived from multivariable conditional logistic regression models. VE was calculated as (1 - OR) × 100%.
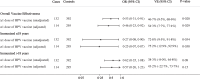
Findings: A total of 524 women (132 cases and 392 controls) were included. The adjusted VE of >1 dose of HPV vaccine was 54% (95% CI: 8-77%, p = 0·03). When the first dose was given at ≤18 years of age VE was 75% (95% CI: 13-93%, p = 0·03), and when vaccinated >18 years VE was 43% (95% CI: -22 to 74%, p = 0·15).
Interpretation: These data demonstrate that the full benefit of HPV vaccines may not be realized when administered at older ages. Continued and strengthened efforts should be made to ensure that recommendations for HPV vaccination of younger adolescents are followed.
Funding: National Institutes of Health, American Cancer Society, Robert E. Leet and Clara Guthrie Patterson Trust, and Centers for Disease Control and Prevention.
Keywords: Case-control study; HPV vaccination; High-grade cervical lesions; Vaccine effectiveness.
© 2025 The Author(s).
Conflict of interest statement
CRO has received grants from National Institutes of Health (NIH), American Cancer Society (ACS), the Robert E. Leet and Clara Guthrie Patterson Trust Foundation, and has leadership roles in the American Academy of Pediatrics-Section on Epidemiology, Public Health, and Evidence (SOEPHE), Eastern Society for Pediatric Research (ESPR), and Journal of the Pediatric Infectious Diseases Society (JPIDS). LMN has received grants from NIH, CDC, served as Scientific Advisor for Merck, GSK, and served on a data safety monitoring board for Moderna and GlaxoSmithKline (GSK). SSS has served as a consultant for Merck, received grants from National Institute of Allergy and Infectious Disease (NIAID), CDC, and National Cancer Institute (NCI), and has leadership role in the American Society for Colposcopy and Cervical Pathology (ASCCP). MKE and EDS have received grant support from the NIH. All other authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

