Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization
- PMID: 41020870
- PMCID: PMC12468322
- DOI: 10.3390/cimb47090748
Resina Draconis Promotes Diabetic Wound Healing by Regulating the AGE-RAGE Pathway to Modulate Macrophage Polarization
Abstract
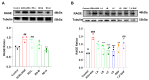
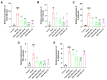
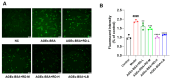
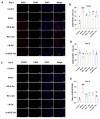
Resina Draconis (RD), a traditional Chinese medicine, has been widely used in treating diabetic foot ulcers. However, its specific mechanisms of action remain incompletely understood. First, network pharmacology combined with GEO clinical sample data mining was employed to systematically analyze the therapeutic targets of RD in promoting diabetic wound healing. Second, an AGEs-induced RAW264.7 cell model was utilized to investigate the regulatory effects of RD and its primary active components on the AGE-RAGE signaling pathway, along with their anti-inflammatory and antioxidant activities. Finally, a diabetic wound mouse model was established to validate the efficacy of RD and further explore its underlying molecular mechanisms. Integrated analysis of network pharmacology and GEO database mining identified 492 potential therapeutic targets of RD in diabetic wound healing, primarily involving the AGE-RAGE pathway. In vitro, RD (6.25 μg/mL) significantly suppressed AGE-induced inflammatory factors and ROS production while downregulating AGE-triggered RAGE protein overexpression. In vivo, RD hydrogel accelerated diabetic wound healing by modulating the AGE-RAGE axis and regulating macrophage polarization. RD effectively promotes diabetic wound healing through synergistic regulation of the AGE-RAGE pathway, oxidative stress suppression, and macrophage polarization modulation, providing a novel therapeutic strategy for diabetic wound management.
Keywords: AGE-RAGE; Resina Draconis; diabetic wound; macrophage polarization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

