Hydrogenation of saturated organic and inorganic molecules in metallic hydrogen
- PMID: 41022716
- PMCID: PMC12479789
- DOI: 10.1038/s41467-025-63552-6
Hydrogenation of saturated organic and inorganic molecules in metallic hydrogen
Abstract
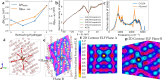
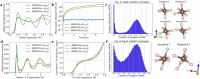
Metallic hydrogen is the most common condensed material in the universe, however, experimental studies are extremely challenging, and understanding of this material has been led by theory. Chemistry in this environment has not been probed experimentally, so here we examine carbon, nitrogen, and oxygen in metallic hydrogen using density functional theory calculations. We find that carbon, nitrogen and oxygen react with each other and metallic hydrogen to produce molecules with covalent-type bonding based on sixfold coordinated carbon, threefold oxygen and fourfold nitrogen: CH6, C2H8, C3H10, OH3, NH4, and CH4OH. In view of the excess hydrogen we refer to them as hypermethane, hyperethane etc. This work suggests that molecular chemistry may take place in very different environments from those found on earth, and may be common throughout the universe. Furthermore, the solubility of C, O, and N casts doubt on whether rocky cores can exist in giant planets.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Wigner, E. & Huntington, H. B. On the possibility of a metallic modification of hydrogen. J. Chem. Phys.3, 764–770 (1935).
-
- Militzer, B., Soubiran, F., Wahl, S. M. & Hubbard, W. Understanding Jupiter’s interior. J. Geophys. Res.121, 1552–1572 (2016).
-
- Nettelmann, N., Becker, A., Holst, B. & Redmer, R. Jupiter models with improved ab initio hydrogen equation of state (H-REOS. 2). Astrophys. J.750, 52 (2012).
-
- Wahl, S. M. & Hubbard, W. B. et al. Comparing Jupiter interior structure models to Juno gravity measurements and the role of a dilute core. Geophys. Res. Lett.44, 4649–4659 (2017).
-
- Loubeyre, P., Occelli, F. & Dumas, P. Synchrotron infrared spectroscopic evidence of the probable transition to metal hydrogen. Nature577, 631–635 (2020). - PubMed
Grants and funding
- EP/P020267/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- Hecate/EC | EC Seventh Framework Programm | FP7 Ideas: European Research Council (FP7-IDEAS-ERC - Specific Programme: "Ideas" Implementing the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007 to 2013))
- CIRRUS/Scottish Funding Council (SFC)
LinkOut - more resources
Full Text Sources

