Development of an adeno-associated virus vector for gene replacement therapy of NF1-related tumors
- PMID: 41022755
- PMCID: PMC12480499
- DOI: 10.1038/s41467-025-63619-4
Development of an adeno-associated virus vector for gene replacement therapy of NF1-related tumors
Abstract
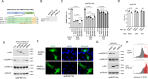
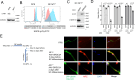
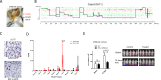
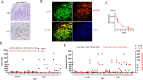
Neurofibromatosis type 1 (NF1) is a tumor predisposition syndrome caused by alterations in NF1 gene that lead to tumor growth throughout the nervous system, which can cause morbidity and mortality, and transform to malignancy. NF1 gene replacement therapy, though promising, is hindered by NF1 gene's large size and delivery challenges. We introduced a membrane-targeted, truncated neurofibromin comprising the GAP-related domain (GRD) fused to the KRAS4B C-terminal domain, which effectively inhibits the RAS signaling pathway and restores Schwann cell differentiation in an NF1 iPSC-derived model. For systemic application, we engineered an adeno-associated virus (AAV) vector using in vivo capsid evolution through sequential DNA shuffling and peptide library screening in a NF1 xenograft mouse model. This tailored vector, AAV-NF, exhibits greatly reduced liver uptake, enhanced tumor targeting across various NF1-related MPNST, neurofibromas and glioma models, and therapeutic efficacy in xenografts of MPNST. This study not only advances a viable AAV vector for NF1 treatment but also outlines a replicable strategy for vector and payload development in other monogenic and tumor-associated disease manifestations.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A patent application on the new AAV vectors for NF1 gene replacement therapy with R.B. and V.S. as co-inventors has been provisionally filed by JHU (63/697,752). The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- W81XWH1810236/United States Department of Defense | United States Army | Army Medical Command | Congressionally Directed Medical Research Programs (CDMRP)
- Distinguished Scientist Award/Sontag Foundation
- 1K08CA230179/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- 5U01CA247576/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

