Isoprene chemistry under upper-tropospheric conditions
- PMID: 41022896
- PMCID: PMC12479858
- DOI: 10.1038/s41467-025-64229-w
Isoprene chemistry under upper-tropospheric conditions
Abstract
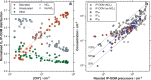
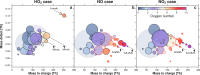
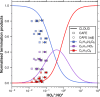
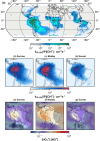
Isoprene (C5H8) is the non-methane hydrocarbon with the highest emissions to the atmosphere. It is mainly produced by vegetation, especially broad-leaved trees, and efficiently transported to the upper troposphere in deep convective clouds, where it is mixed with lightning NOx. Isoprene oxidation products drive rapid formation and growth of new particles in the tropical upper troposphere. However, isoprene oxidation pathways at low temperatures are not well understood. Here, in experiments at the CERN CLOUD chamber at 223 K and 243 K, we find that isoprene oxygenated organic molecules (IP-OOM) all involve two successive oxidations. However, depending on the ambient concentrations of the termination radicals ( , and ), vastly-different IP-OOM emerge, comprising compounds with zero, one or two nitrogen atoms. Our findings indicate high IP-OOM production rates for the tropical upper troposphere, mainly resulting in nitrate IP-OOM but with an increasing non-nitrate fraction around midday, in close agreement with aircraft observations.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Guenther, A. B. et al. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev.5, 1471–1492 (2012).
-
- Ying, Q., Li, J. & Kota, S. H. Significant contributions of isoprene to summertime secondary organic aerosol in Eastern United States. Environ. Sci. Technol.49, 7834–7842 (2015). - PubMed
-
- Pressley, S. et al. Long-term isoprene flux measurements above a northern hardwood forest. J. Geophys. Res. Atmos.110, D07301 (2005).
-
- Potosnak, M. J. et al. Isoprene emissions from a tundra ecosystem. Biogeosciences10, 871–889 (2013).
-
- Wennberg, P. O. et al. Gas-phase reactions of isoprene and its major oxidation products. Chem. Rev.118, 3337–3390 (2018). - PubMed
LinkOut - more resources
Full Text Sources

