Prevention of type 2 diabetes through prediabetes remission without weight loss
- PMID: 41023486
- PMCID: PMC12532634
- DOI: 10.1038/s41591-025-03944-9
Prevention of type 2 diabetes through prediabetes remission without weight loss
Abstract
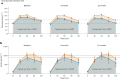
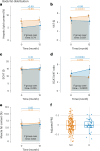
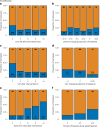
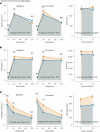
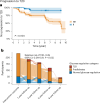
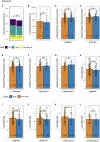
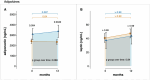
Clinical practice guidelines recommend defined weight loss goals for the prevention of type 2 diabetes (T2D) in those individuals with increased risk, such as prediabetes. However, achieving prediabetes remission, that is, reaching normal glucose regulation according to American Diabetes Association criteria, is more efficient in preventing T2D than solely reaching weight loss goals. Here we present a post hoc analysis of the large, multicenter, randomized, controlled Prediabetes Lifestyle Intervention Study (PLIS), demonstrating that prediabetes remission is achievable without weight loss or even weight gain, and that it also protects against incident T2D. The underlying mechanisms include improved insulin sensitivity, β-cell function and increments in β-cell-GLP-1 sensitivity. Weight gain was similar in those achieving prediabetes remission (responders) compared with nonresponders; however, adipose tissue was differentially redistributed in responders and nonresponders when compared against each other-while nonresponders increased visceral adipose tissue mass, responders increased adipose tissue in subcutaneous depots. The findings were reproduced in the US Diabetes Prevention Program. These data uncover essential pathways for prediabetes remission without weight loss and emphasize the need to include glycemic targets in current clinical practice guidelines to improve T2D prevention.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.K. receives grants from the German Diabetes Association, Wilhelm Doerenkamp Foundation and California Walnut Commission, and lecture fees from Sanofi, Boehringer Ingelheim, Berlin Chemie, Lilly Deutschland and Juzo Akademie. R.W. receives lecture fees from Eli Lilly, Novo Nordisk and Sanofi, and participates in a Data Safety Monitoring Board and Advisory Board for Eli Lilly. N.P. receives lecture fees from Novo Nordisk and Gesellschaft für Wissenschaftstransfer Dresden, advisory board fees from Bayer Vital and holds patent WO2021/092265A1 for diagnosis and treatment of nonalcoholic fatty liver disease and liver fibrosis. J.J.H. is consulting with Novo Nordisk. N.J.W.A. has received research support and speaker fees from Mercodia, Novo Nordisk, Merck/MSD and Boehringer Ingelheim. All other authors declare no competing interests.
Figures

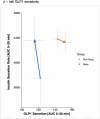

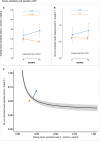
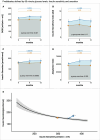

References
-
- Sandforth, L. et al. Prediabetes remission to reduce the global burden of type 2 diabetes. Trends Endocrinol. Metab.S1043-2760, 00004-9 (2025). - PubMed
-
- Ligthart, S. et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol.4, 44–51 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

