Performance evaluation of a point of care capillary blood test for C-reactive protein in community healthcare
- PMID: 41023849
- PMCID: PMC12482497
- DOI: 10.1186/s12875-025-02972-1
Performance evaluation of a point of care capillary blood test for C-reactive protein in community healthcare
Abstract
Background: Antibiotics are often prescribed without a C-Reactive Protein (CRP) level, an inflammatory marker used to guide treatment decisions, due to delays in obtaining laboratory results. We evaluated a novel point of care testing (POCT) device that measures CRP within minutes using a small capillary blood sample obtained via finger prick, in community healthcare settings.
Method: Patients in the community having blood samples taken for CRP measurement provided finger prick capillary blood in children and venous blood in adults, who also provided an additional capillary sample. All capillary samples were tested using the LumiraDX™ POCT device, which provided a quantitative CRP measurement within four minutes. Venous samples from adults and the same capillary samples from children were then tested using a standard laboratory method.
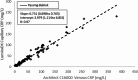
Results: A total of 161 patients agreed to measurements by the two methods. CRP levels as assessed by the laboratory method ranged from 0.9 to 382 mg/L, and 5.3 to 232.1 mg/L by POCT. There was a good correlation between results of the standard laboratory method and the POCT assay for CRP (r = 0.97), with a mean bias of -11.3 (slope 0.73, intercept 3.9 mg/L). Of the 161 patients tested, 57 gave CRP results outside the limits of quantitation of the POCT assay and were not included in the regression and bias analysis.
Conclusion: The LumiraDX™ POCT device demonstrates sufficient accuracy in measuring CRP levels from finger prick capillary blood. The availability of this technology across community healthcare settings will improve the assessment of infection and support more precise antibiotic prescribing.
Trial registration: ACCom/SE/202,324/01 (Date of registration 17/08/2023).
Keywords: CRP; Capillary blood sample; Community; Finger prick; Point of care.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This investigation was defined as a service evaluation by Oxford Health NHS Foundation Trust Clinical Audit Team. Our Trust policy dictate that service evaluations are considered by the Clinical Audit Team and approved or modified by that team. Ethical committee approval is sought only when the Clinical Audit Team considers it appropriate. In the case of this investigation, the (Clinical Audit committee) Clinical Audit Team approved it as a service evaluation not requiring ethical committee approval or formal written consent from potential participants (ACCom/SE/2023-24/01). All participants, and parents of participants under the age of 16, provided informed verbal consent prior to participation, in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. 10.1056/NEJM199902113400607. - PubMed
-
- Van den Bruel A, Thompson MJ, Haj-Hassan T, Stevens R, Moll H, Lakhanpaul M, Mant D. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ. 2011;342:d3082. 10.1136/bmj.d3082. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous