Neoadjuvant therapy with eribulin, doxorubicin and cyclophosphamide for patients with HER2-negative inflammatory breast cancer: a phase II study
- PMID: 41024110
- PMCID: PMC12482238
- DOI: 10.1186/s13058-025-02108-4
Neoadjuvant therapy with eribulin, doxorubicin and cyclophosphamide for patients with HER2-negative inflammatory breast cancer: a phase II study
Abstract
Background: Inflammatory breast cancer (IBC) is an aggressive and highly angiogenic disease. Eribulin is a microtubule inhibitor with anti-angiogenic properties.
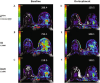
Methods: In a phase II trial, we examined the efficacy of an eribulin-containing neoadjuvant regimen (eribulin- > doxorubicin plus cyclophosphamide (AC) or AC- > eribulin) for patients with newly diagnosed HER2-negative IBC. Pathologic complete response (pCR: ypT0/Tis ypN0) was the primary endpoint; residual cancer burden (RCB) categories were also recorded. Five patients from each cohort underwent dynamic contrast enhanced MRI (DCE-MRI) and diffusion weighted MRI. All patients had research breast biopsies for transcriptomic, differential gene expression, and cell subset analysis at baseline and one week after the first dose of therapy.
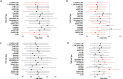
Results: 19/22 (86.4%) patients had hormone receptor-positive disease. All patients were able to undergo planned curative-intent surgery and radiation. One patient had a pCR, and long-term outcomes were encouraging: after median follow up of 76 months, 3 patients experienced disease recurrence. Five-year event-free survival (EFS) was 85.6%. The regimen was tolerated with expected side effects-the most common grade 1 or 2 AEs were fatigue (95.5%), nausea (68.2%), and alopecia (63.6%). Seven out of 22 (31.8%) patients experienced any grade 3 or 4 AE, with neutropenia (22.7%) being the most common. DCE-MRI showed decreased tumor vascularization after 1 week of treatment versus baseline. Transcriptomic analysis using quantification of synthesized dsDNA libraries and tumor microenvironment analysis of paired baseline and on-treatment samples showed residual cancer burden (RCB)-III tumors were more likely to have genes associated with adipogenesis/fatty acid metabolism and cells associated with immunosuppression.
Conclusions: Despite the low pCR rate, all patients were able to undergo curative surgery, and long-term outcomes were encouraging with 5-year EFS 85.6%. Decreases in tumor vascularization with treatment were detected by DCE-MRI parameters irrespective of initial chemotherapy received. Adipogenesis/fatty acid metabolism and cells associated with immunosuppression are potential mechanisms of resistance and targets for future investigation in this unique patient population.
Trial registration: ClinicalTrials.gov (NCT02623972)(Registration date: 12/02/15).
Keywords: Cyclophosphamide; Doxorubicin; Eribulin; HER2-negative; Inflammatory Breast Cancer; Neoadjuvant therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Standards and the Declaration of Helsinki. Institutional review board approval was obtained at Dana-Farber/Harvard Cancer Center (DF/HCC). Monitoring of trial progress and safety was reviewed twice yearly by the DF/HCC independent data safety monitoring committee (DSMC). All patients provided written informed consent prior to initiating any study treatments or procedures. This trial was registered with ClinicalTrials.gov (NCT02623972). Consent for publication: Not applicable. Competing interests: BTH reports spousal employment at Eli Lilly. JB reports serving as an educational speaker for Varian; royalties from UpToDate; and honorarium from Oncoclinicas and Physician's Education Resource. JLG reports serving as a consultant for Glaxo-Smith Kline (GSK), Codagenix, Verseau Therapeutics, Kymera, Kowa, Duke Street Bio, and Array BioPharma; and receives sponsored research support from Duke Street Bio, GSK, Array BioPharma, Merck and Eli Lilly. SMT reports serving in a consulting or advisory role for Novartis, Pfizer (SeaGen), Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb/Systimmune, Daiichi Sankyo, Gilead, Blueprint Medicines, Reveal Genomics, Sumitovant Biopharma, Artios Pharma, Menarini/Stemline, Aadi Bio, Bayer, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Effector, Hengrui USA, Cullinan Oncology, Circle Pharma, Arvinas, BioNTech, Johnson&Johnson/Ambrx, Launch Therapeutics, Zuellig Pharma, Bicycle Therapeutics, BeiGene Therapeutics, Mersana, Summitt Therapeutics, Avenzo Therapeutics, Aktis Oncology, Celcuity, Boehringer Ingelheim, Samsung Bioepis, and Olema Pharmaceuticals; research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, AstraZeneca, Gilead, NanoString Technologies, Seattle Genetics, OncoPep, Daiichi Sankyo, Menarini/Stemline, and Jazz Pharmaceuticals; and travel support from Eli Lilly, Sanofi, Gilead, Jazz Pharmaceuticals, Pfizer Arvinas, Roche, Sanofi, and Olema Pharmaceuticals. MR reports research funding/resources (to institution) from Bayer, Biotheranostics, Bristol-Myers Squibb, Novartis, Pfizer, Roche, TerSera Therapeutics, and ETOP IBCSG Partners Foundation; and consulting/advisory roles or honorarium for AstraZeneca, Bristol-Myers Squibb, TerSera Therapeutics, and Tolmar Pharmaceuticals. BO reports clinical trial support from Eisai Pharmaceuticals and Incyte Pharmaceuticals. FL reports research funding to the institution from AstraZeneca, Zentalis, Ideaya, Gilead, Merck, and Incyte; and advisory/consulting roles for AstraZeneca, Pfizer, Eli Lilly, and Daiichi Sankyo. The remaining authors declare no competing interests.
Figures

References
-
- Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008;26(5):786–90. - PubMed
-
- Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10(23):7965–71. - PubMed
-
- Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, et al. Combined-modality treatment of inflammatory breast carcinoma: twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40(4):321–9. - PubMed
-
- Baldini E, Gardin G, Evagelista G, Prochilo T, Collecchi P, Lionetto R. Long-term results of combined-modality therapy for inflammatory breast carcinoma. Clin Breast Cancer. 2004;5(5):358–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

