Enhancing Methods for Research on Cannabis: A Workshop Report
- PMID: 41024165
- PMCID: PMC12482547
- DOI: 10.1186/s42238-025-00314-7
Enhancing Methods for Research on Cannabis: A Workshop Report
Abstract
Aims: Progressive legalization of medical and recreational cannabis markets at the state-level has led to rapid growth of medical and recreational cannabis markets and to product diversification with emerging products having high concentrations of delta-9-tetrahydrocannabinol. Research on these products is still limited and the evidence available for policy formulation is diminished by methodological limitations.
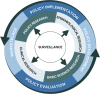
Methods: As a step towards addressing these limitations, the Colorado School of Public Health convened a multidisciplinary workshop that addressed four areas of cannabis research: epidemiological, clinical, surveillance, and policy. Workshop participants provided recommendations in each area to advance research on cannabis to make it more informative for decision-making on key policy topics. Emphasis was placed on assessment of use of cannabis products by study participants.
Results: Recommendations for research methods and their implementation were made in the four areas. Those for epidemiology include using a core set of exposure assessment measures across three domains; developing this core set through a national and/or international scientific consensus process; ensuring the core set of measures are validated and readily available; and updating the core set periodically to account for ongoing changes in the cannabis landscape. Recommendations in the clinical research area include standard dosing and dosing terminology; standardized data collection instruments; identifying biomarkers for detecting cannabis exposure; and biological matrices. Policy research recommendations were offered for state regulators, evaluators/researchers, and policy makers. Surveillance recommendations include developing and implementing a novel and nimble surveillance system to monitor use of high-concentration forms of cannabis; adding questions to existing surveillance systems with the objective of monitoring high-concentration cannabis and adverse outcomes; and elevating the coordination, synthesis, and dissemination of findings in existing data sources that could signal adverse outcomes from high-concentration cannabis.
Conclusions: Given the changing marketplace, it is urgent to improve the informativeness of cannabis research through enhanced research methods.
Keywords: Clinical research; Delta-9-tetrahydrocannabinol; Dose; Epidemiological research; Exposure; High-concentration cannabis; Policy; Public health; Surveillance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not Applicable. Consent for publication: Not Applicable. Competing interest: The authors declare no competing interests.
Figures
References
-
- American College of Medical Toxicology. Toxicology Investigators Consortium (ToxIC) Homepage Phoenix, AZ. 2024 [Available from: https://www.acmt.net/core-registry/. Accessed 10 May 2025.
-
- Berl V, Hurd YL, Lipshutz BH, Roggen M, Mathur EJ, Evans M. A Randomized, Triple-Blind, Comparator-Controlled Parallel Study Investigating the Pharmacokinetics of Cannabidiol and Tetrahydrocannabinol in a Novel Delivery System, Solutech, in Association with Cannabis Use History. Cannabis Cannabinoid Res. 2022;7(6):777–89. - PMC - PubMed
-
- Bialas P, Fitzcharles MA, Klose P, Hauser W. Long-term observational studies with cannabis-based medicines for chronic non-cancer pain: A systematic review and meta-analysis of effectiveness and safety. Eur J Pain. 2022;26(6):1221–33. - PubMed
-
- Cannabis Research and Policy Project Team. A Scoping Review on Health Effects of High-Concentration Cannabis Products: Findings on Key Policy Questions. Colorado School of Public Health; 2023. Contract No.: 76.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials