Optic nerve sheath diameter for prediction of intracranial hypertension after ischemic sTrokE - The ONSITE study
- PMID: 41024460
- PMCID: PMC12484050
- DOI: 10.1177/23969873251379985
Optic nerve sheath diameter for prediction of intracranial hypertension after ischemic sTrokE - The ONSITE study
Abstract
Background: Intracranial hypertension (IH) from brain edema is a life-threatening complication of large vessel occlusion (LVO) stroke, yet clinical monitoring is often unreliable. Non-invasive methods for early IH prediction are needed. This study assessed whether sonographic measurement of the optic nerve sheath diameter (ONSD) could improve the prediction of IH after stroke.
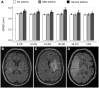
Patients and methods: We prospectively measured the internal optic nerve sheath diameter (ONSDint) via transorbital ultrasound in 65 stroke patients and 30 controls. ONSD was also measured on the initial CT or MRI. The primary endpoint of IH was a composite of clinical and radiological signs of brain swelling. A predictive ONSD cut-off was determined from a multivariable logistic regression model, adjusted for age and infarct volume. Predictive performance was assessed using leave-one-out cross-validation.
Results: Seven of 65 stroke patients (11%) developed IH. The initial sonographic ONSDint was significantly increased in patients who developed IH. The multivariable model identified an optimal predictive cut-off of ⩾5.51 mm, which predicted IH with a sensitivity of 85.7% and a specificity of 94.8%. In comparison, ONSD derived from initial neuroimaging was also a strong predictor, with an optimal cut-off of 6.80 mm yielding a sensitivity of 100% and a specificity of 91.1%, and showed superior predictive accuracy in the cross-validation (AUC 0.905 vs 0.687).
Discussion: Our sonographic ONSDint cut-off of ≥5.51 mm aligns well with recent stroke literature that used similar standardized measurement techniques. Our findings also highlight the distinct roles of different imaging modalities. While the initial CT/MRI provides a static measurement with high predictive power, the unique advantage of sonography is its bedside applicability, allowing for the crucial, non-invasive serial monitoring of ONSD as a dynamic marker of intracranial pressure changes.
Conclusion: Early ONSD assessment is a valuable predictor of IH after severe stroke. A sonographic ONSDint of ⩾5.51 mm identifies patients at high risk with excellent accuracy. While initial neuroimaging may offer superior predictive power, bedside sonography remains a crucial, repeatable tool for monitoring these critically ill patients.
Keywords: Ischemic stroke; intracranial hypertension; intracranial pressure; large vessel occlusion stroke; malignant media infarction; optic nerve sheath; optic nerve sheath diameter; ultrasound.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PB: Research funds by the Gottfried and Julia Bangerter-Rhyner Foundation and Swiss Academy of Medical Sciences and funding for travel and conference fees from BMS/Pfizer and Novo Nordisk. SW: received research funds by the Swiss National Science Foundation, the UZH Clinical research priority program (CRPP) stroke, the Zurich Neuroscience Center (ZNZ), the Baugarten foundation, the Hartmann Müller Foundation, the Koetser Foundation, the Swiss Heart Foundation; and speaker honoraria from Amgen, Springer, Advisis AG, Teva Pharma, Boehringer Ingelheim, Lundbeck, Astra Zeneca, FoMF, and a consultancy fee from Bayer and Novartis via institution for research.
Figures





References
-
- Hofmeijer J, Algra A, Kappelle LJ, et al. Predictors of life-threatening brain edema in middle cerebral artery infarction. Cerebrovasc Dis 2008; 25: 176–184. - PubMed
-
- Hacke W, Schwab S, Horn M, et al. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. - PubMed
-
- Heiss W-D. Malignant MCA infarction: pathophysiology and imaging for early diagnosis and management decisions. Cerebrovasc Dis 2016; 41: 1–7. - PubMed
-
- Wijdicks EF, Sheth KN, Carter BS, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 1222–1238. - PubMed
-
- Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch Neurol 1984; 41: 26–29. - PubMed
LinkOut - more resources
Full Text Sources

