Targeting glypican-3 as a new frontier in liver cancer therapy
- PMID: 41024885
- PMCID: PMC12476774
- DOI: 10.4254/wjh.v17.i9.107671
Targeting glypican-3 as a new frontier in liver cancer therapy
Abstract
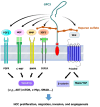
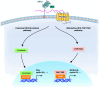
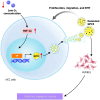
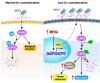
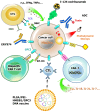
Glypican-3 (GPC3) is a tumor-associated antigen that is specifically expressed in hepatocellular carcinoma (HCC) and having relatively low levels in normal tissues. This unique expression pattern positions GPC3 as a potential target for precision therapy and drug development in HCC. Recent studies have shown significant advancements in GPC3-targeted therapies and immunotherapies, particularly for patients with advanced or treatment-resistant HCC. Although certain clinical trials have yielded suboptimal results, numerous ongoing studies continue to explore its therapeutic efficacy. This mini-review focuses on the latest research developments regarding GPC3 as a therapeutic target across various HCC treatment strategies, including monoclonal antibodies, bispecific antibodies, chimeric antigen receptor-T-cell therapies, and other innovative approaches. In addition, the limitations of GPC3-targeted therapies and their future application prospects in HCC treatment are discussed. The review particularly emphasizes the unmet need for future research directions, such as combination immunotherapy strategies and novel drug designs. Through the integration of innovative technologies and clinical validation, GPC3 holds strong potential as a promising breakthrough in the treatment of HCC, offering new opportunities for enhancing patient outcomes and improving therapeutic efficacy.
Keywords: Chimeric antigen receptor T-cell; Glypican-3; Hepatocellular carcinoma; Immunotherapy; Wnt.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest related to this manuscript.
Figures
References
-
- Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129:899–906. - PubMed
-
- Lin H, Huber R, Schlessinger D, Morin PJ. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res. 1999;59:807–810. - PubMed
-
- Xiang YY, Ladeda V, Filmus J. Glypican-3 expression is silenced in human breast cancer. Oncogene. 2001;20:7408–7412. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

