Evidence-based standardized sample handling protocol for accurate blood-based Alzheimer's disease biomarker measurement: Results and consensus of the Global Biomarker Standardization Consortium
- PMID: 41025225
- PMCID: PMC12481164
- DOI: 10.1002/alz.70752
Evidence-based standardized sample handling protocol for accurate blood-based Alzheimer's disease biomarker measurement: Results and consensus of the Global Biomarker Standardization Consortium
Abstract
Introduction: Blood-based biomarkers (BBMs) have revolutionized Alzheimer's disease diagnosis and monitoring. Their pre-analytical stability requires scrutiny. This study assessed pre-analytical effects to inform a standardized sample handling protocol.
Methods: Assessed pre-analytical variations included collection tube type, hemolysis, centrifugation settings, centrifugation/storage delays, tube transfers, and freeze-thawing (n = 15/experiment). Phosphorylated tau (pTau) isoforms were measured with Simoa, Lumipulse, MesoScale Discovery, and immunoprecipitation-mass spectrometry. Amyloid-beta (Aβ42, Aβ40), glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) protein were measured with Simoa.
Results: All assessed BBM levels varied by over 10% by collection tube type. Aβ peptides were the most sensitive, and their levels declined >by more than 10% under storage and centrifugation delays, more steeply at room temperature (RT) compared with 2°C to 8°C. NfL and GFAP levels increased by more than 10% upon RT/-20°C storage. pTau isoforms demonstrated stability across most pre-analytical variations.
Discussion: We established an evidence-based handling protocol to ensure reliable sample handling for neurological BBMs upon adoption in clinics, trials, and research.
Highlights: Sample handling protocols can mitigate pre-analytical effects on BBM results. We developed an evidence-based, expert-consensus plasma sample handling protocol. Primary collection tube and delays to centrifuging or freezing impact AD BBMs. Plasma pTau217 is highly resistant to pre-analytical sample handling variations. Plasma Aβ42 and Aβ40 were most sensitive to pre-analytical variations.
Keywords: amyloid beta; glial fibrillary acidic protein; neurofilament light; phosphorylated tau; plasma biomarkers; pre‐analytical variability; pre‐analytics; sample handling; stability.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M.G., D.A.B., M.vL., B.B., I.H., and S.J. have nothing to disclose. IV has consultancy contracts with Quanterix and Neurogen Biomarking, which are paid directly to her institution. I.V. performs contract research for Nitrase Therapeutics and Roche Diagnostics, which is paid directly to her institution. W.F. has been funded by ZonMW, NWO, EU‐JPND, EU‐IHI, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health Holland, Topsector Life Sciences & Health, stichting Dioraphte, Noaber Foundation, Pieter Houbolt Fonds, Gieskes‐Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, Philips, Biogen MA Inc., Novartis‐NL, Life‐MI, AVID, Roche BV, Lilly Nederland, Fujifilm, Eisai, Combinostics. W.F. holds the Pasman Chair. W.F. is the recipient of ABOARD, which is a public–private partnership receiving funding from ZonMW (No. 73305095007) and Health Holland, Topsector Life Sciences & Health (PPP‐allowance; No. LSHM20106) and is the recipient of TAP‐dementia (
Figures



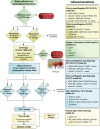
References
-
- Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21:66‐77. - PubMed
-
- Schindler SE, Galasko D, Pereira AC, et al. Acceptable performance of blood biomarker tests of amyloid pathology—recommendations from the Global CEO Initiative on Alzheimer's Disease. Nat Rev Neurol. 2024;20:426‐439. - PubMed
MeSH terms
Substances
Grants and funding
- research grants of Health Holland and ZonMW
- Pasman Stichting. Research of Alzheimer Center Amsterdam
- Stichting Alzheimer Nederland and Stichting Steun Alzheimercentrum Amsterdam
- 2017-00915/Swedish Research Council
- 2022-00732/Swedish Research Council
- AF-930351/Swedish Alzheimer Foundation
- AF-939721/Swedish Alzheimer Foundation
- AF-968270/Swedish Alzheimer Foundation
- AF-994551/Swedish Alzheimer Foundation
- ALZ2022-0006/Hjärnfonden, Sweden
- FO2024-0048-TK-130/Hjärnfonden, Sweden
- FO2024-0048-HK-24/Hjärnfonden, Sweden
- European Union Joint Program for Neurodegenerative Disorders
- Alzheimer's Association 2021 Zenith Award
- La Fondation Recherche Alzheimer (FRA)
- Kirsten and Freddy Johansen Foundation
- Swedish State Support for Clinical Research
- ADSF-21-831376-C/AD Strategic Fund and the Alzheimer's Association
- ADSF-21-831381-C/AD Strategic Fund and the Alzheimer's Association
- ADSF-21-831377-C/AD Strategic Fund and the Alzheimer's Association
- ADSF-24-1284328-C/AD Strategic Fund and the Alzheimer's Association
- the European Partnership on Metrology
- Cure Alzheimer's Fund
- Olav Thon Foundation, the Erling-Persson Family Foundation
- Familjen Rönströms Stiftelse
- Familjen Beiglers Stiftelse
- Stiftelsen för Gamla Tjänarinnor
- 860197/Marie Skłodowska-Curie
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre
- 860197 (MIRIADE/European Commission (Marie Curie International Training Network
- 101119596/European Commission (Marie Curie International Training Network
- Innovative Medicines Initiatives 3TR
- (bPRIDE, CCAD), European Partnership on Metrology
- 101053962/European Union's Horizon Europe Research and Innovation Programme
- 101053962/European Union's Horizon Europe Research and Innovation Programme
- 201809-2016862/the Alzheimer Drug Discovery Foundation
- Michael J. Fox Foundation
- Health Holland, the Dutch Research Council (ZonMW)
- Alzheimer Drug Discovery Foundation
- Selfridges Group Foundation
- Alzheimer Netherlands
- Health Holland
- LSHM20106/Topsector Life Sciences & Health
- Dutch National Dementia Strategy
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

