Drug-Induced Periocular and Ocular Surface Disorders: An EAACI Position Paper
- PMID: 41025570
- PMCID: PMC12590328
- DOI: 10.1111/all.70074
Drug-Induced Periocular and Ocular Surface Disorders: An EAACI Position Paper
Abstract
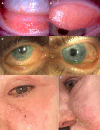
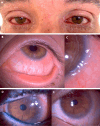
Various systemic and topical medications can induce ocular and periocular cutaneous adverse effects (AEs), ranging from mild to severe. These AEs may lead to ocular surface (OS) damage and, in some cases, life-threatening complications. Drug-induced ocular adverse reactions are generally classified into two primary categories: toxic reactions and/or allergic hypersensitivity reactions, which can be IgE or non-IgE-mediated. Systemic antibiotics, antivirals, and anticonvulsants can trigger adverse reactions that may involve the OS. Drugs like antihistamines, beta-blockers, antipsychotics, antidepressants, and isotretinoin are linked to dry eye disease. Topical treatments-including antibiotics, antiglaucoma medications, preservatives, contact lens solutions, and cosmetics-may elicit allergic or toxic ocular diseases. Recent evidence implicates ocular surface AEs in patients undergoing biological treatments for oncological diseases and atopic dermatitis. Epidermal growth factor receptor inhibitors, used in the treatment of several cancers, have been associated with conjunctivitis, meibomitis, dry eye, periocular skin changes, and trichomegaly. Similarly, dupilumab, the first biologic approved for treating moderate-to-severe atopic dermatitis, has also been linked to OS disease with blepharoconjunctivitis. This position paper provides a comprehensive overview of the clinical presentations, diagnostic approaches, and treatment strategies for drug-induced ocular AEs, integrating the latest literature and clinical guidelines.
Keywords: biological treatments; drug related blepharoconjunctivitis; ocular allergy; ocular drug hypersensitivity reactions; severe cutaneous adverse reactions.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Andrea Leonardi: Consultancy for Bausch & Lomb, Dompè, FAES Farma, FIDIA, Santen, Théa, SIFI. Christophe Baudouin: Consultancy for Bausch & Lomb, Glaukos, Horus Pharma, Oculis, Santen, and Thea. Vibha Sharma: Consultancy for DBV, Novartis. Jasper Therapeutics. Serge Doan: Received honoraria from Alcon, Almirall, Bausch & Lomb, Horus, Leo Pharma, Sanofi, Santen, Thea. All other authors declare no conflicts of interest.
Figures


References
-
- Dona I., Torres M. J., Celik G., Phillips E., Tanno L. K., and Castells M., “Changing Patterns in the Epidemiology of Drug Allergy,” Allergy 79, no. 3 (2024): 613–628. - PubMed
-
- Brockow K., Ardern‐Jones M. R., Mockenhaupt M., et al., “EAACI Position Paper on How to Classify Cutaneous Manifestations of Drug Hypersensitivity,” Allergy 74, no. 1 (2019): 14–27. - PubMed
-
- Leonardi A., Bogacka E., Fauquert J. L., et al., “Ocular Allergy: Recognizing and Diagnosing Hypersensitivity Disorders of the Ocular Surface,” Allergy 67, no. 11 (2012): 1327–1337. - PubMed
-
- Jutel M., Agache I., Zemelka‐Wiacek M., et al., “Nomenclature of Allergic Diseases and Hypersensitivity Reactions: Adapted to Modern Needs: An EAACI Position Paper,” Allergy 78, no. 11 (2023): 2851–2874. - PubMed
-
- “Correction to: Nomenclature of Allergic Diseases and Hypersensitivity Reactions: Adapted to Modern Needs: An EAACI Position Paper,” Allergy 79, no. 1 (2024): 269–273. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

