Novel Role of Copper Transporter CTR1 and Therapeutic Potential of Copper Chelators in Retinal Ischemia-Reperfusion Injury
- PMID: 41025873
- PMCID: PMC12489862
- DOI: 10.1167/iovs.66.12.70
Novel Role of Copper Transporter CTR1 and Therapeutic Potential of Copper Chelators in Retinal Ischemia-Reperfusion Injury
Abstract
Purpose: Retinal ischemia contributes to vision loss in ischemic and diabetic retinopathies through oxidative stress, neurovascular injury, and inflammation. Copper (Cu), whereas an essential micronutrient, can be toxic in excess and is regulated by Cu transporters such as CTR1. However, the role of CTR1 in ischemic retinopathy remains unclear.
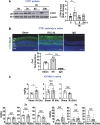
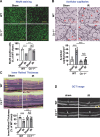
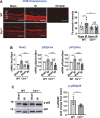
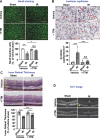
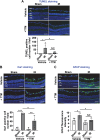
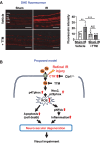
Methods and results: Retinal ischemia-reperfusion (IR) injury was induced by elevating intraocular pressure (IOP) to 110 millimeters of mercury (mm Hg) for 40 minutes in the right eyes of Ctr1 heterozygous (Ctr1+/-) and wild-type (WT) mice. In WT mice, IR triggered rapid CTR1 upregulation and increased retinal Cu levels (measured by inductively coupled plasma mass spectrometry [ICP-MS]). IR injury caused retinal ganglion cell (RGC) loss, inner retinal thinning, vascular degeneration, and apoptosis, all of which were significantly attenuated in Ctr1+/- mice. Ctr1+/- mice also exhibited reduced microglial (Iba1⁺) and glial cells (GFAP⁺) activation and preserved visual function, as assessed by electroretinography. Mechanistically, IR-induced reactive oxygen species (\({{\rm{O}}_{2}}^{-}\)) production (DHE staining), upregulation of NADPH oxidase components (NOX2 and p47phox), and NF-κB activation were markedly suppressed in Ctr1+/- mice. Treatment with the Cu chelator tetrathiomolybdate (TTM) similarly reduced retinal thinning, neurovascular damage, apoptosis, gliosis, and oxidative stress after IR injury.
Conclusions: CTR1 plays a central role in mediating Cu-dependent oxidative stress, neurovascular degeneration, and inflammation following retinal IR injury. Targeting the CTR1-Cu axis may represent a novel therapeutic strategy for ischemic retinopathy.
Conflict of interest statement
Disclosure:
Figures


Update of
-
Novel Role of Copper Transporter CTR1 and Therapeutic Potential of Copper Chelators in Retinal Ischemia-Reperfusion Injury.bioRxiv [Preprint]. 2025 May 21:2025.05.17.654687. doi: 10.1101/2025.05.17.654687. bioRxiv. 2025. Update in: Invest Ophthalmol Vis Sci. 2025 Sep 2;66(12):70. doi: 10.1167/iovs.66.12.70. PMID: 40475640 Free PMC article. Updated. Preprint.
References
-
- Osborne NN, Casson RJ, Wood JPM, Chidlow G, Graham M, Melena J.. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004; 23(1): 91–147. - PubMed
-
- Cuenca N, Fernández-Sánchez L, Campello L, et al.. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014; 43: 17–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

