Brain Frailty and Functional Outcomes After Thrombolysis for Acute Ischemic Stroke
- PMID: 41026485
- PMCID: PMC12485648
- DOI: 10.1001/jamanetworkopen.2025.34941
Brain Frailty and Functional Outcomes After Thrombolysis for Acute Ischemic Stroke
Abstract
Importance: The cumulative burden of chronic vascular and neurodegenerative changes contributes to brain frailty, which may reduce the brain's capacity to recover from acute ischemic stroke (AIS). The association between brain frailty markers and poststroke outcomes after thrombolysis is unclear.
Objective: To evaluate associations between brain frailty assessed on non-contrast-enhanced computed tomography (NCCT) and magnetic resonance imaging (MRI) and functional outcome in patients with AIS treated with intravenous thrombolysis.
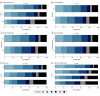
Design, setting, and participants: This cohort study was a post hoc analysis of the Alteplase compared to Tenecteplase (AcT) trial, an investigator-led, registry-linked, parallel-group, open-label, randomized clinical trial that enrolled patients between December 10, 2019, and January 25, 2022, at 22 primary and comprehensive stroke centers across Canada. Participants included adults 18 years or older diagnosed with ischemic stroke causing disabling neurological deficit, presenting within 4.5 hours of symptom onset, and meeting Canadian guidelines for thrombolysis. Markers of brain frailty (cortical and subcortical atrophy, white matter changes [Fazekas score, grouped as 0, 1-2, and 3-6], lacunes, chronic infarctions, and [on MRI] microbleeds, siderosis, and enlarged perivascular spaces) were retrospectively assessed while reviewers were blinded to outcome variables. Analyses were performed from July 24, 2024, to March 25, 2025.
Exposures: Patients underwent baseline NCCT and were randomized to receive intravenous thrombolysis with alteplase (0.9 mg/kg) or tenecteplase (0.25 mg/kg). Some patients also received posttreatment brain MRI.
Main outcomes and measures: The primary outcome was excellent functional outcome (modified Rankin Scale [mRS] score of 0-1) at 90 days. Secondary outcomes included 90-day ordinal mRS score (trichotomized as 0-2, 3-4, and 5-6), symptomatic intracerebral hemorrhage, and mortality. Sensitivity analyses were performed in patients with available MRI scans.
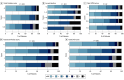
Results: Among the 1568 patients (median age, 74 [IQR, 63-83] years; 817 male [52.1%]) with interpretable NCCT findings, after correcting for multiple comparisons, higher total Fazekas score of 3 to 6 compared with 0 was associated with lower odds of a 90-day mRS score of 0 to 1 (adjusted odds ratio [OR], 0.40 [95% CI, 0.24-0.65]). Total Fazekas score (adjusted common OR [ACOR], 2.80 [95% CI, 1.88-4.16]), cortical atrophy (ACOR, 2.65 [95% CI. 1.63-4.32]), and total brain frailty score (ACOR, 3.15 [95% CI, 1.87-5.33]) were each associated with worse ordinal mRS, but were not associated with safety outcomes.
Conclusions and relevance: In this cohort study of patients with AIS treated with intravenous thrombolysis, brain frailty markers-particularly white matter changes, cortical atrophy, and total brain frailty-were associated with worse outcomes. Consideration of these neuroimaging markers may better inform clinicians and patients about treatment expectations from thrombolytic therapy.
Conflict of interest statement
Figures


Comment in
References
-
- IST-3 Collaborative Group . Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol. 2015;14(5):485-496. doi: 10.1016/S1474-4422(15)00012-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

