Distal mutations enhance catalysis in designed enzymes by facilitating substrate binding and product release
- PMID: 41027962
- PMCID: PMC12484545
- DOI: 10.1038/s41467-025-63802-7
Distal mutations enhance catalysis in designed enzymes by facilitating substrate binding and product release
Abstract
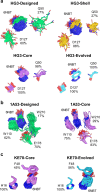
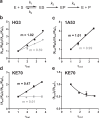
The role of amino-acid residues distant from an enzyme's active site in facilitating the complete catalytic cycle-including substrate binding, chemical transformation, and product release-remains poorly understood. Here, we investigate how distal mutations promote the catalytic cycle by engineering mutants of three de novo Kemp eliminases containing either active-site or distal mutations identified through directed evolution. Kinetic analyses, X-ray crystallography, and molecular dynamics simulations reveal that while active-site mutations create preorganized catalytic sites for efficient chemical transformation, distal mutations enhance catalysis by facilitating substrate binding and product release through tuning structural dynamics to widen the active-site entrance and reorganize surface loops. These distinct contributions work together to improve overall activity, demonstrating that a well-organized active site, though necessary, is not sufficient for optimal catalysis. Our findings reveal critical roles that distal residues play in shaping the catalytic cycle to enhance efficiency, yielding valuable insights for enzyme design.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
Distal mutations enhance catalysis in designed enzymes by facilitating substrate binding and product release.bioRxiv [Preprint]. 2025 Feb 27:2025.02.21.639315. doi: 10.1101/2025.02.21.639315. bioRxiv. 2025. Update in: Nat Commun. 2025 Sep 30;16(1):8662. doi: 10.1038/s41467-025-63802-7. PMID: 40060566 Free PMC article. Updated. Preprint.
References
-
- Du, S. et al. Conformational ensembles reveal the origins of serine protease catalysis. Science387, eado5068 (2025). - PubMed
MeSH terms
Substances
Grants and funding
- P30 GM124169/GM/NIGMS NIH HHS/United States
- RGPAS-2021-00017/Gouvernement du Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada)
- 26503/Canada Foundation for Innovation (Fondation canadienne pour l'innovation)
- R35 GM145238/GM/NIGMS NIH HHS/United States
- R01 GM124149/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

