Radiomics-enhanced modelling approach for predicting the need for ECMO in ARDS patients: a retrospective cohort study
- PMID: 41028296
- PMCID: PMC12485099
- DOI: 10.1038/s41598-025-21287-w
Radiomics-enhanced modelling approach for predicting the need for ECMO in ARDS patients: a retrospective cohort study
Abstract
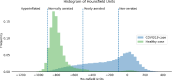
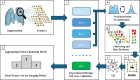
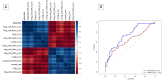
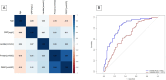
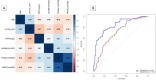
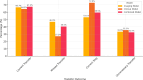
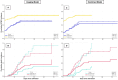
Decisions regarding veno-venous extracorporeal membrane oxygenation (vv-ECMO) in patients with acute respiratory distress syndrome (ARDS) are often based solely on clinical and physiological parameters, which may insufficiently reflect severity and heterogeneity of lung injury. This study aimed to develop a predictive model integrating machine learning-derived quantitative features from admission chest computed tomography (CT) with selected clinical variables to support early individualized decision-making regarding vv-ECMO therapy. In this retrospective single-center cohort study, 375 consecutive patients with COVID-19-associated ARDS admitted to the ICU between March 2020 and April 2022 were included. Lung segmentation from initial CTs was performed using a convolutional neural network (CNN) to generate high-resolution, anatomically accurate masks of the lungs. Subsequently, 592 radiomic features, quantifying lung aeration, density and morphology, were extracted. Four clinical parameters - age, mean airway pressure, lactate, and C-reactive protein, were selected on the basis of clinical relevance. Three logistic regression models were developed: (1) Imaging Model, (2) Clinical Model, and (3) Combined Model integrating different features. Predictive performance was assessed via the area under the receiver operating characteristic curve (AUROC), accuracy, sensitivity, and specificity. A total of 375 patients were included: 172 in the training and 203 in the validation cohort. In the training cohort, the AUROCs were 0.743 (Imaging), 0.828 (Clinical), and 0.842 (Combined). In the validation cohort, the Combined Model achieved the highest AUROC (0.705), outperforming the Clinical (0.674) and Imaging (0.639) Models. Overall accuracy in the validation cohort was 64.0% (Combined), 66.5% (Clinical), and 59.1% (Imaging). The Combined Model showed 68.1% sensitivity and 58.9% specificity. Kaplan-Meier analysis confirmed a significantly greater cumulative incidence of ECMO therapy in patients predicted as high risk (p < 0.001), underscoring its potential to support individualized, timely ECMO decisions in ARDS by providing clinicians with objective data-driven risk estimates. Quantitative CT features based on machine learning-derived lung segmentation allow early individualized prediction of the need for vv-ECMO in ARDS. While clinical data remain essential, radiomic markers enhance prognostic accuracy. The Combined Model demonstrates considerable potential to support timely and evidence-based ECMO initiation, facilitating individualized critical care in both specialized and general ICU environments.Trial registration: The study is registered with the German Clinical Trials Register under the number DRKS00027856. Registered 18.01.2022, retrospectively registered due to retrospective design of the study.
Keywords: CNN; CT; Computed tomography; Convolutional neural network; Critical care; Decision making; ECMO; Extracorporeal membrane oxygenation; Lung segmentation; Prediction; Radiomic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and approved by the responsible Ethics Committee of the Technical University of Dresden, Germany (BO-EK-374072021).
Figures





References
-
- Combes, A. et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med.378 (21), 1965–1975 (2018). - PubMed
-
- Bos, L. D. J. & Ware, L. B. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet400 (10358), 1145–1156 (2022). - PubMed
-
- Gorman, E. A., O’Kane, C. M. & McAuley, D. F. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet400 (10358), 1157–1170 (2022). - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

