A co-culture system to study the effects of Poly I:C-activated microglia on the differentiation of murine primary neural stem cells
- PMID: 41028599
- PMCID: PMC12589351
- DOI: 10.1007/s11626-025-01091-6
A co-culture system to study the effects of Poly I:C-activated microglia on the differentiation of murine primary neural stem cells
Abstract
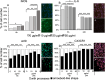
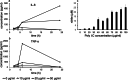
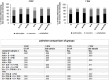
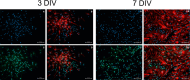
Studies in rodents have shown that systemic inflammation induced by prenatal exposure to the viral mimetic polyinosinic:polycytidylic acid (Poly I:C) triggers maternal immune activation. Cytokines released by the maternal immune system can cross the placenta and enter fetal circulation. In the fetal brain, embryonic microglia may produce additional cytokines and other inflammatory mediators in response to maternally derived cytokines. This resulting cytokine imbalance is suggested to impair neurogenesis and brain development, potentially contributing to the onset of neuropsychiatric disorders in offspring. To investigate microglial involvement in neurogenesis under pathological conditions, we used the spontaneously immortalized microglial cell line (SIM-A9), and confirmed the expression of Iba1 and CD68 via immunocytochemistry. Additionally, SIM-A9 cells expressed CX3CR1, Ki67, and isolectin. Upon Poly I:C stimulation, SIM-A9 cells released the cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), as well as nitric oxide (NO), as determined by ELISA and Griess assay, respectively. After confirming SIM-A9 cell activation by Poly I:C, we co-cultured these cells with neural stem/progenitor cells (NSPCs) from embryonic mouse neocortex using a transwell system. We examined how chronically activated microglia influence NSPC differentiation and characterized the resulting cell phenotypes using immunocytochemistry. Our results demonstrate that SIM-A9 cells support NSPC differentiation into neurons as early as three days in culture. However, the number of neurons decreased with prolonged culture. Furthermore, Poly I:C in the NSPC culture media, as well as cytokines secreted by Poly I:C-activated SIM-A9 cells, showed a supportive effect on astrocyte differentiation.
Keywords: Co-culture; Microglia; Neuronal stem cells; Poly I:C.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All experiments were performed according to the principles regarding the care and use of animals adopted by the German Animal Welfare Law for the prevention of cruelty to animals after approval by the LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz, Nordrhine-Westfalia). This article does not contain any studies involving human participants performed by any of the authors. Conflicts of interest: The authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- Ajmone-Cat MA, Nicolini A, Minghetti L (2003) Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signaling pathways and selectively promotes prostaglandin E2 synthesis. J Neurochem 87(5):1193–1203. 10.1046/j.1471-4159.2003.02087.x - PubMed
-
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO (2008) Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 26(9):2361–2371. 10.1634/stemcells.2007-0914 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

