Genetic tuning of retinal ganglion cell subtype identity to drive visual behavior
- PMID: 41028711
- PMCID: PMC12484735
- DOI: 10.1038/s41467-025-63675-w
Genetic tuning of retinal ganglion cell subtype identity to drive visual behavior
Abstract
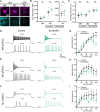
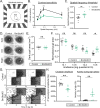
The distinct blend of molecular and cellular features that define neuronal subtype identity are central to shaping how individual subtypes impact animal behavior. The diversity of the mammalian nervous system is vast - the retina alone contains over 100 neuronal subtypes. Yet, the genetic processes giving rise to this stunning structural and functional diversity remain poorly understood. Here, we uncover a graded expression pattern of the transcription factor BRN3B that tunes and maintains multiple, subtype-defining transcriptional and morphophysiological features of the melanopsin-expressing, intrinsically photosensitive retinal ganglion cells (ipRGCs) in mice. Disruption of BRN3B expression levels causes the transcriptional and morphophysiological identity of ipRGC subtypes to begin to converge, leading to dysfunction in multiple ipRGC-dependent behaviors. These findings show that graded levels of a single transcription factor can tune a diverse array of features to shape neuronal identity and circuit function to drive behavior.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.D.B. is a co-founder of Aura Life Science, in which he has a financial interest. All other authors declare that they have no competing interests.
Figures



Update of
-
Genetic tuning of intrinsically photosensitive retinal ganglion cell subtype identity to drive visual behavior.bioRxiv [Preprint]. 2024 Apr 28:2024.04.25.590656. doi: 10.1101/2024.04.25.590656. bioRxiv. 2024. Update in: Nat Commun. 2025 Sep 30;16(1):8678. doi: 10.1038/s41467-025-63675-w. PMID: 38712084 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- EY025202/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- EY027983/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- R01 NS123285/NS/NINDS NIH HHS/United States
- R01 EY034662/EY/NEI NIH HHS/United States
- DA053691/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

