This is a preprint.
SNARE mimicry by the CD225 domain of IFITM3 enables regulation of homotypic late endosome fusion
- PMID: 41030966
- PMCID: PMC12478428
- DOI: 10.1101/2024.08.07.607021
SNARE mimicry by the CD225 domain of IFITM3 enables regulation of homotypic late endosome fusion
Update in
-
SNARE mimicry by the CD225 domain of IFITM3 enables regulation of homotypic late endosome fusion.EMBO J. 2025 Jan;44(2):534-562. doi: 10.1038/s44318-024-00334-8. Epub 2024 Dec 9. EMBO J. 2025. PMID: 39653855 Free PMC article.
Abstract
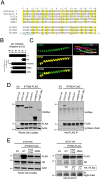
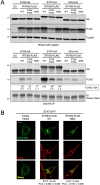
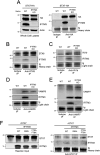
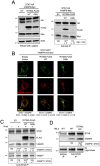
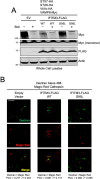
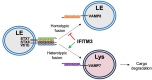
The CD225/Dispanins superfamily consists of membrane proteins that regulate vesicular transport and membrane fusion events driving neurotransmission, glucose transport, and antiviral immunity. However, how the CD225 domain controls membrane trafficking was unknown. We reveal that the CD225 domain contains a SNARE-like motif that enables interaction with cellular SNARE fusogens. Proline rich transmembrane protein 2 (PRRT2) encodes a SNARE-like motif that enables interaction with neuronal SNARE proteins, and mutations therein disrupt SNARE binding and are linked to neurological disease. Another CD225 member, interferon-induced transmembrane protein 3 (IFITM3), protects cells against Influenza A virus infection. IFITM3 interacts with SNARE proteins that mediate late endosome-late endosome (homotypic) fusion and late endosome-lysosome (heterotypic) fusion. IFITM3 binds to syntaxin 7 (STX7) in cells and in vitro, and mutations that abrogate STX7 binding cause loss of antiviral activity against Influenza A virus. Mechanistically, IFITM3 disrupts assembly of the SNARE complex controlling homotypic fusion and accelerates the trafficking of endosomal cargo to lysosomes. Our results suggest that SNARE modulation plays a previously unrecognized role in the diverse functions performed by CD225 proteins.
Conflict of interest statement
Disclosure and Competing Interests Statement The authors declare no competing interests.
Figures


References
-
- Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR (2002) Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol 9: 107–111 - PubMed
-
- Beaton N, Rudigier C, Moest H, Müller S, Mrosek N, Röder E, Rudofsky G, Rülicke T, Ukropec J, Ukropcova B et al. (2015) TUSC5 regulates insulin-mediated adipose tissue glucose uptake by modulation of GLUT4 recycling. MOLMET 4: 795–810
-
- Becker F, Schubert J, Striano P, Anttonen AK, Liukkonen E, Gaily E, Gerloff C, Muller S, Heussinger N, Kellinghaus C et al. (2013) PRRT2-related disorders: further PKD and ICCA cases and review of the literature. J Neurol 260: 1234–1244 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
