This is a preprint.
Symptomatic treatment by a BBB-permeable AAV engineered to restore TDP-43 function slows motor neuron disease and prevents paralysis
- PMID: 41030970
- PMCID: PMC12478394
- DOI: 10.1101/2025.08.14.670400
Symptomatic treatment by a BBB-permeable AAV engineered to restore TDP-43 function slows motor neuron disease and prevents paralysis
Abstract
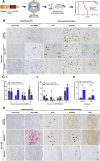
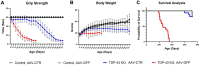
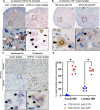
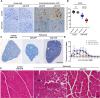
TAR DNA-binding protein 43kDa (TDP-43) dysfunction is an early pathogenic mechanism that underlies amyotrophic lateral sclerosis (ALS), a devastating neurodegenerative disorder that lacks disease modifying therapies. We previously developed a mouse model in which TDP-43 is selectively deleted from motor neurons (ChAT-Cre;Tardbp f/f ) that mimics the early stages of ALS. Here, we demonstrate that intravenous delivery of a blood-brain-barrier (BBB) permeable AAV capsid expressing our rationally designed splicing repressor CTR (AAV-PHP.eB-CTR) in symptomatic ChAT-Cre;Tardbp f/f mice markedly slowed disease progression and prevented paralysis. Systemic delivery of AAV-PHP.eB-CTR led to transduction of ~80% of spinal motor neurons, repression of TDP-43-associated cryptic exons within motor neurons expressing CTR, and attenuation of motor neuron loss. Notably, the addition of the TARDBP 3'UTR autoregulatory element to CTR maintained its expression within a physiological range. In control littermates that received AAV-PHP.eB-CTR and were monitored for >20 months, grip strength and body weight remained normal, and no histopathological abnormalities were observed, underscoring a favorable safety profile for this gene therapy. These results provide preclinical proof-of-concept that BBB-crossing AAV delivery of CTR can rescue motor neuron disease through the restoration of TDP-43 function, offering a promising mechanism-based therapeutic strategy for ALS.
Keywords: Amyotrophic Lateral Sclerosis; Blood-brain-barrier permeable AAV; Cryptic exons; Gene therapy; Motor neuron; Splicing repressor; Symptomatic treatment; TDP-43 autoregulatory element; TDP-43 dysfunction.
Figures
References
-
- Arnold E. S., Ling S.-C., Huelga S. C., Lagier-Tourenne C., Polymenidou M., Ditsworth D., Kordasiewicz H. B., McAlonis-Downes M., Platoshyn O., Parone P. A., Da Cruz S., Clutario K. M., Swing D., Tessarollo L., Marsala M., Shaw C. E., Yeo G. W., & Cleveland D. W. (2013). ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proceedings of the National Academy of Sciences of the United States of America, 110(8), E736–745. 10.1073/pnas.1222809110 - DOI - PMC - PubMed
-
- Baughn M. W., Melamed Z., López-Erauskin J., Beccari M. S., Ling K., Zuberi A., Presa M., Gonzalo-Gil E., Maimon R., Vazquez-Sanchez S., Chaturvedi S., Bravo-Hernández M., Taupin V., Moore S., Artates J. W., Acks E., Ndayambaje I. S., Agra de Almeida Quadros A. R., Jafar-Nejad P., … Cleveland D. W. (2023). Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies. Science (New York, N.Y.), 379(6637), 1140–1149. 10.1126/science.abq5622 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
