This is a preprint.
Regulation of airway fumarate by host and pathogen promotes S. aureus pneumonia
- PMID: 41030993
- PMCID: PMC12478345
- DOI: 10.1101/2025.04.10.647998
Regulation of airway fumarate by host and pathogen promotes S. aureus pneumonia
Update in
-
Regulation of airway fumarate by host and pathogen promotes Staphylococcus aureus pneumonia.Nat Commun. 2025 Aug 1;16(1):7050. doi: 10.1038/s41467-025-62453-y. Nat Commun. 2025. PMID: 40745169 Free PMC article.
Abstract
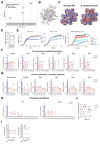
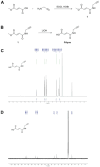
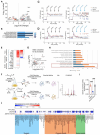
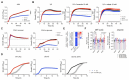
Staphylococcus aureus is a leading cause of healthcare-associated pneumonia, contributing significantly to morbidity and mortality worldwide. As a ubiquitous colonizer of the upper respiratory tract, S. aureus must undergo substantial metabolic adaptation to achieve persistent infection in the distinctive microenvironment of the lung. We observed that fumC, which encodes the enzyme that converts fumarate to malate, is highly conserved with low mutation rates in S. aureus isolates from chronic lung infections. Fumarate, a pro-inflammatory metabolite produced by macrophages during infection, is regulated by the host fumarate hydratase (FH) to limit inflammation. Here, we demonstrate that fumarate, which accumulates in the chronically infected lung, is detrimental to S. aureus, blocking primary metabolic pathways such as glycolysis and oxidative phosphorylation (OXPHOS). This creates a metabolic bottleneck that drives staphylococcal FH (FumC) activity for airway adaptation. FumC not only degrades fumarate but also directs its utilization into critical pathways including the tricarboxylic acid (TCA) cycle, gluconeogenesis and hexosamine synthesis to maintain metabolic fitness and form a protective biofilm. Itaconate, another abundant immunometabolite in the infected airway enhances FumC activity, in synergy with fumarate. In a mouse model of pneumonia, a ΔfumC mutant displays significant attenuation compared to its parent and complemented strains, particularly in fumarate- and itaconate-replete conditions. Our findings underscore the pivotal role of immunometabolites in promoting S. aureus pulmonary adaptation.
Conflict of interest statement
Competing interests Robert Sebra is a consultant and equity holder for GeneDx and a founder and equity holder of Panacent Bio. All other authors declare no competing interests.
Figures


References
-
- Rumpf C, Lange J, Schwartbeck B, Kahl BC. Staphylococcus aureus and Cystic Fibrosis-A Close Relationship. What Can We Learn from Sequencing Studies? Pathogens. 2021;10(9).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
