This is a preprint.
Identification of SLC45A4 as a pain gene encoding a neuronal polyamine transporter
- PMID: 41031034
- PMCID: PMC12478382
- DOI: 10.1101/2024.09.25.615046
Identification of SLC45A4 as a pain gene encoding a neuronal polyamine transporter
Update in
-
SLC45A4 is a pain gene encoding a neuronal polyamine transporter.Nature. 2025 Oct;646(8084):404-412. doi: 10.1038/s41586-025-09326-y. Epub 2025 Aug 20. Nature. 2025. PMID: 40836097 Free PMC article.
Abstract
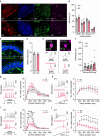
Polyamines are regulatory metabolites with key roles in transcription, translation, cell signalling and autophagy1. They are implicated in multiple neurological disorders including stroke, epilepsy and neurodegeneration and can regulate neuronal excitability through interactions with ion channels2. Polyamines have been linked to pain showing altered levels in human persistent pain states and modulation of pain behaviour in animal models3. However, the systems governing polyamine transport within the nervous system remain unclear. In undertaking a Genome Wide Association Study (GWAS) of chronic pain intensity in the UK-Biobank we found significant association with variants mapping to the SLC45A4 gene locus. In the mouse nervous system SLC45A4 expression is enriched in all sensory neuron sub-types within the dorsal root ganglion including nociceptors. Cell-based assays show that SLC45A4 is a selective plasma membrane polyamine transporter, whilst the cryo-EM structure reveals a novel regulatory domain and basis for polyamine recognition. Mice lacking SLC45A4 show normal mechanosensitivity but reduced sensitivity to noxious heat and algogen induced tonic pain that is associated with reduced excitability of peptidergic nociceptors. Our findings thus establish a role for neuronal polyamine transport in pain perception and identify a new target for therapeutic intervention in pain treatment.
Conflict of interest statement
Competing interests DLB has acted as a consultant for 5 am ventures, AditumBio , Astra Zeneca, Biogen, Biointervene, Combigene, GSK, Ionis, Lexicon therapeutics, Neuvati, Novo Ventures, Olipass, Orion, Replay, SC Health Managers, Third Rock ventures, Vida Ventures, Vertexon behalf of Oxford University Innovation over the last 2 years. The PAINSTORM consortium received funding from Eli Lilly and Astra Zenca. The DOLORisk consortium received funding from Eli Lilly. JEL is an employee of AstraZeneca. HRK is a member of advisory boards for Altimmune, Clearmind Medicine, Dicerna Pharmaceuticals, Enthion Pharmaceuticals, and Sophrosyne Pharmaceuticals; a consultant to Sobrera Pharmaceuticals and Altimmune; the recipient of research funding and medication supplies for an investigator-initiated study from Alkermes; a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the last three years by Alkermes, Dicerna, Ethypharm, Lundbeck, Mitsubishi, Otsuka, and Pear Therapeutics; and a holder of U.S. patent 10,900,082 titled: “Genotype-guided dosing of opioid agonists,” issued 26 January 2021.
Figures



References
Main References
-
- Sagar N. A., Tarafdar S., Agarwal S., Tarafdar A. & Sharma S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med Sci (Basel) 9 (2021). 10.3390/medsci9020044 - DOI
-
- Liu J., Yu Z., Maimaiti B., Meng Q. & Meng H. The Potential Role of Polyamines in Epilepsy and Epilepsy-Related Pathophysiological Changes. Biomolecules 12 (2022). 10.3390/biom12111596 - DOI
References for methods:
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
