Evaluation of the Safety, Pharmacokinetics, and Antitumor Activity of Tusamitamab Ravtansine in Patients With Nonsquamous NSCLC With High or Moderate Expression of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 5
- PMID: 41031047
- PMCID: PMC12478256
- DOI: 10.1016/j.jtocrr.2025.100844
Evaluation of the Safety, Pharmacokinetics, and Antitumor Activity of Tusamitamab Ravtansine in Patients With Nonsquamous NSCLC With High or Moderate Expression of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 5
Abstract
Introduction: Tusamitamab ravtansine is an antibody-drug conjugate targeting cells expressing carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) with a maytansinoid payload, DM4. This phase 1b dose-expansion study (NCT02187848) evaluated its safety, pharmacokinetics, and preliminary antitumor activity in patients with nonsquamous NSCLC (NSq NSCLC).
Methods: Patients aged above or equal to 18 years with advanced or metastatic NSq NSCLC, life expectancy more than or equal to 12 weeks, and high (≥2+ intensity in ≥50% of tumor cells) or moderate (≥2+ intensity in 1%-49% of tumor cells) CEACAM5 expression (assessed by immunohistochemistry) received intravenous tusamitamab ravtansine 100 mg/m2 every 2 weeks.
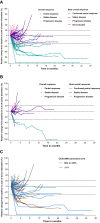
Results: A total of 64 patients with high and 28 with moderate CEACAM5 expression received a median of 8.0 (1-69) and 4.5 (1-38) treatment cycles, respectively. High expressors had 13 confirmed partial responses and 28 stable diseases (objective response rate, 20.3%; 95% confidence interval [CI]: 12.3%-31.7%, p < 0.0001); median duration of response was 6.7 months, and median time to progression was 3.7 months (95% CI: 2.7-5.1 mo). Moderate expressors had two confirmed partial responses (objective response rate, 7.1%; 95% CI: 2.0%-22.7%, p = 0.4117) and 15 stable diseases.Treatment-emergent adverse events (AEs) occurred in 78.3% of patients (72/92), 37.0% (34/92) of patients required dose modifications, and 5.4% (5/92) discontinued treatment. The most common treatment-emergent AEs included asthenia (37.0%), keratitis (29.3%), and dyspnea (23.9%). Corneal AEs occurred in 38.0% (35/92), typically grade 1/2, reversible, and manageable by dose modifications.
Conclusions: Tusamitamab ravtansine demonstrated a favorable safety profile, objective responses, and antitumor activity in patients with high CEACAM5-expressing NSq NSCLC.
Keywords: Antibody-drug conjugate; Carcinoembryonic antigen-related cell adhesion molecule 5; Non–small cell lung cancer; Phase 1 study; Tusamitamab ravtansine.
© 2025 by the International Association for the Study of Lung Cancer.
Conflict of interest statement
Dr. Gazzah has received travel, accommodation, and congress registration expenses from Boehringer Ingelheim, Novartis, Pfizer, Roche, and Sanofi; has served in a consultant/expert role for Novartis; has served as principal/subinvestigator of clinical trials for Aduro Biotech, Agios Pharmaceuticals, Amgen, Argenx BVBA, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, AVEO, Bayer Healthcare AG, BBB Technologies BV, BeiGene, BioAlliance Pharma, BioNTech AG, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, CA, Celgene Corporation, Chugai Pharmaceutical Co., Clovis Oncology, Daiichi Sankyo, Debiopharm S.A., Eisai, Exelixis, Forma, GamaMabs, Genentech, Inc., Gilead Sciences, Inc., GlaxoSmithKline, Glenmark Pharmaceuticals, H3 Biomedicine, Inc., Hoffmann-La Roche AG, Incyte Corporation, Innate Pharma, IRIS Servier, Janssen, Kura Oncology, Kyowa Kirin Pharm, Lilly, Loxo Oncology, Lytix Biopharma AS, MedImmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar Therapeutics, Novartis Pharma, OCTIMET Oncology NV, OncoEthix, OncoMed, Oncopeptides, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Pfizer, PharmaMar, Pierre Fabre, Rigontec GmbH, Roche, Sanofi Aventis, Sierra Oncology, Taiho Pharmaceutical, Tesaro, Inc., Tioma Therapeutics, Inc., and Xencor; has received research grants from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, and Sanofi; and reports receiving nonfinancial support (drug supplied) from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Johnson & Johnson, Lilly, MedImmune, Merck, NH TherAguix, Pfizer, and Roche. Dr. Ricordel has received funding from Roche and Novartis and reports that their institution has received funding from AstraZeneca, 10.13039/100007013F. Hoffmann-La Roche, Bristol Myers Squibb, 10.13039/100004336Novartis, and Pfizer. Dr. Italiano reports that their institution has served in a consulting or advisory role for Bayer, Merck Sharp & Dohme, Merck, Roche, Parthenon, Bristol Myers Squibb, PharmaMar, and Novartis; and reports that their institution has received research funding from 10.13039/100004326Bayer, 10.13039/100009947Merck Sharp & Dohme, Merck, Roche, Parthenon, Bristol Myers Squibb, PharmaMar, and Novartis. Dr. Cho is an employee of Yonsei University Health System; reports stock/ownership interests in TheraCanVac Inc., Gencurix Inc., Bridge Biotherapeutics, Kanaph Therapeutics, Cyrus Therapeutics, Interpark Bio Convergence Corp., and J INTS BIO; has served in a consulting or advisory role for Abion, BeiGene, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol Myers Squibb, CJ Bioscience, Curelogen, Cyrus Therapeutics, ONO, Onegene Biotechnology, Yuhan, Pfizer, Eli Lilly, GI Cell, Guardant Health, HK inno.N, Imnewrun Biosciences Inc., Janssen, Takeda, Merck Sharp & Dohme, MedPacto, Blueprint Medicines, RandBio, and Hanmi; has served as member of the board of directors for Interpark Bio Convergence Corp. and J INTS BIO; has served on advisory boards for Kanaph Therapeutics, Bridge Biotherapeutics, Cyrus Therapeutics, Guardant Health, and Oscotec Inc.; has patents, royalties, and other intellectual property with Champions Oncology, Crown Bioscience, and Imagen; has served as an invited speaker for ASCO, AstraZeneca, Guardant Health, Roche, ESMO, IASLC, Korean Cancer Association, Korean Society of Medical Oncology, Korean Society of Thyroid-Head and Neck Surgery, Korean Cancer Study Group, Novartis, Merck Sharp & Dohme, The Chinese Thoracic Oncology Society, and Pfizer; has other relationships with DAAN Biotherapeutics; and reports their institution has received research funding from MOGAM Institute for Biomedical Research, LG Chem, Oscotec, Interpark Bio Convergence Corp, GI Innovation, GI Cell, Abion, AbbVie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Oncology, CJ Bioscience, CJ Blossom Park, Cyrus Therapeutics, Dizal Pharma, Genexine, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Nuvalent, Oncternal, ONO, Regeneron, Dong-A ST, Bridge Biotherapeutics, Yuhan, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, J INTS BIO, Hanmi, and CHA Bundang Medical Center. Dr. Calvo is an employee of START and HM Hospitales; has served in a consulting or advisory role for Nanobiotix, Janssen-Cilag, PsiOxus Therapeutics, Seattle Genetics, Roche/Genentech, Amcure, TargImmune Therapeutics, Servier, Bristol Myers Squibb, PharmaMar, Alkermes, Amunix, Adcendo, Anaveon, AstraZeneca/MedImmune, BeiGene, Chugai Pharma, MonTa, MEDSIR, MSD Oncology, Nouscom, Novartis, OncoDNA, Sanofi, Syneos Health, T-Knife, and Boehringer Ingelheim; holds a leadership role in START; has other relationship with Investigational Therapeutics in Oncological Sciences; has stock/ownership interests in START and Oncoart Associated; reports honoraria from HM Hospitales; has received research funding from 10.13039/100017239BeiGene and START; and reports their institution has received research funding from Achilles Therapeutics. Dr. Kim has served in a consulting or advisory role for Amgen, AstraZeneca, Bristol Myers Squibb/ONO Pharmaceuticals, Daiichi Sankyo, GlaxoSmithKline, Janssen, Merck, Merck Sharp & Dohme, Oncobix, Pfizer, SK Biopharm, and Takeda; and has received medical writing support from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chong Kun Dang, Daiichi Sankyo, GlaxoSmithKline, Pfizer, Merck Sharp & Dohme, Merck, Novartis, Roche, Takeda, and Yuhan; and reports their institution has received research funding from Alpha Biopharma, Amgen, AstraZeneca/MedImmune, Boehringer Ingelheim, Bristol Myers Squibb, Bridge Biotherapeutics, Chong Keun Dang, Daiichi Sankyo, GlaxoSmithKline, Hanmi, Janssen, Merck, Merus, Mirati Therapeutics, Merck Sharp & Dohme, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, Turning Point Therapeutics, Xcovery, Yuhan, and inno.N. Dr. Helissey has served in a consulting or advisory role for Janssen, Astellas, Bayer, Merck Sharp & Dohme, AstraZeneca, and Viatris. Dr. Kim Jin-Soo has served in a consulting or advisory role for IMBdx; has received research funding from AstraZeneca, Merck, AbbVie, Eli Lilly, Boryung, BeiGene, Sanofi-Aventis, Merck Sharp & Dohme, Yuhan, HK inno.N, Daiichi Sankyo, ALX Oncology, ONO, Chong Keun Dang, Roche, and Gencurix; and reports honoraria from Boryung, Sanofi-Aventis, Gencurix, and GeneCker. Dr. Vieito has served in a consulting or advisory role for Bristol Myers Squibb and Roche; reports their institution has received research funding from Novartis, Roche, Thermo Fisher, AstraZeneca, Taiho, and BeiGene; and reports PI studies with Novartis, Roche, Bristol Myers Squibb, Laminar Pharma, Taiho, Incyte, PharmaMar, and Sanofi. Dr. Moreno is an employee of START; has served in a consulting or advisory role for Roche, Bayer, Bristol Myers Squibb, Janssen, Syneos, Affimed, and AstraZeneca; has served in a speakers bureau for Pierre Fabre, Janssen, and Bayer; and reports PI studies/institutional funding with AbbVie, ACEA Biosciences, Adaptimmune, ADC Therapeutics, Aduro, Agenus, Amcure, Amgen, Astellas, AstraZeneca, Bayer, BeiGene, BioInvent International AB, Bristol Myers Squibb, Boehringer Ingelheim, Boston Pharmaceuticals, Celgene, Daichii-Sankyo, Debiopharm, Eisai, e-Therapeutics, Exelixis, Forma Therapeutics, Genmab, GlaxoSmithKline, Harpoon Therapeutics, Hutchison, Immutep, Incyte, Inovio, Iovance, Janssen, Kyowa Kirin, Lilly, Loxo, MEDSIR, Menarini, Merck, Merus, Millennium, Merck Sharp & Dohme, Nanobiotix, Nektar, Novartis, Odonate Therapeutics, Pfizer, PharmaMar, Principia, PsiOxus, Puma, Regeneron, Rigontec, Roche, Sanofi, Sierra Oncology, Synthon, Taiho, Takeda, Tesaro, Transgene, Turning Point Therapeutics, and Upsher-Smith Laboratories. Dr. Paz-Ares has served in a consulting or advisory role for Roche, Merck Sharp & Dohme, Merck Serono, Bristol Myers Squibb, AstraZeneca, Lilly, Pfizer, PharmaMar, Bayer, Amgen, Janssen, GlaxoSmithKline, Novartis, Takeda, Sanofi, Mirati Therapeutics, BeiGene, Daichii, Medscape, and PER; reports speaker fees from Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Lilly, PharmaMar, BeiGene, Daichii-Sankyo, Medscape, and PER; was an invited speaker for Amgen; has other relationships with Genomica, Altum Sequencing, AACR, ASCO, AECC, ASEICA, ESMO, Oncosur, and Small Cell Lung Cancer Group; and reports their institution received funding from Daichii-Sankyo, AstraZeneca, Merck Sharp & Dohme Corp., Bristol Myers Squibb, Janssen-Cilag International, Novartis, Roche, Sanofi, Tesaro, Alkermes, Lilly, Takeda, Pfizer, and PharmaMar. Ms. Masson is an employee of IT&M Stats on behalf of Sanofi. Dr. Barlesi reports institutional financial interests with AbbVie, ACEA, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd., Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, MedImmune, Merck, Merck Sharp & Dohme, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda. Dr. Fagniez, Dr. Chadjaa, Dr. Bauchet, and Dr. Soufflet are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Ghiringhelli and Dr. Cousin declare no conflicts of interest.
Figures


References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Duma N., Santana-Davila R., Molina J.R. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. - PubMed
-
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Nadler E., Espirito J.L., Pavilack M., Boyd M., Vergara-Silva A., Fernandes A. Treatment patterns and clinical outcomes among metastatic non-small-cell lung cancer patients treated in the community practice setting. Clin Lung Cancer. 2018;19:360–370. - PubMed
LinkOut - more resources
Full Text Sources

