Sex-specific genetic effects on susceptibility to idiopathic pulmonary fibrosis
- PMID: 41031098
- PMCID: PMC12477485
- DOI: 10.1183/23120541.00200-2025
Sex-specific genetic effects on susceptibility to idiopathic pulmonary fibrosis
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a chronic lung condition that is more prevalent in males than females. The reasons for this are not fully understood; differing environmental exposures due to historically sex-biased occupations and diagnostic bias are possible explanations. To date, over 20 independent genetic association signals have been reported for IPF susceptibility, but these have been discovered when combining males and females. The objectives of the present study were to assess whether there is a need to consider sex-specific effects when evaluating genetic risk in clinical prediction models for IPF and to test for sex-specific associations with IPF susceptibility.
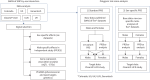
Methods: We performed a genome-wide single nucleotide polymorphism (SNP)-by-sex interaction study meta-analysis of IPF risk in six independent case-control studies comprising 4561 cases (1280 females, 3281 males) and 22 888 controls (8360 females, 14 528 males) of European genetic ancestry. We used polygenic risk scores (PRSs) comprising common (minor allele frequency >1%) autosomal variants to assess differences in genetic risk prediction between males and females.
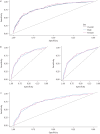
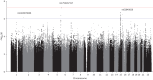
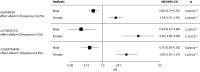
Results: The predictive accuracy of the PRSs were similar between males and females, regardless of whether using combined or sex-specific association results. Three new independent genetic association signals were identified (p<1×10-6).
Conclusions: The predictive accuracy of common autosomal SNP-based PRSs did not vary significantly between males and females. We prioritised three genetic variants whose effect on IPF risk may be modified by sex. These findings would not account for the differences in prevalence between males and females. Future studies should ensure adequate representation of both sexes.
Copyright ©The authors 2025.
Conflict of interest statement
Conflicts of interest: A. Adengunsoye declares funding from the National Institutes of Health (NIH) (K23HL146942); consulting fees from Genentech, Inogen, Medscape, AbbVie, patientMpower and Boehringer Ingelheim; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer Ingelheim. A.D. Stockwell is a full-time employee of Genentech/Roche with stock and stock options in Roche. A.F. Goesman was a full-time employee of Pharmaceutical Product Development (PPD), part of Thermo Fisher Scientific, until June 2023. B. Guillen-Guio declares fellowship funding from the Wellcome Trust (221680/Z/20/Z). B.L. Yaspan is a full-time employee of Genentech/Roche with stock and stock options in Roche. C. Flores declares funding from Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III and Instituto Tecnológico y de Energías Renovables; honoraria in educational events from Fundación Instituto Roche. D.A. Schwartz declares being the founder and chief scientific officer of Eleven P15, Inc., a company dedicated to the early diagnosis and treatment of pulmonary fibrosis. F. Martinez declares funding from the (US) National Heart, Lung, and Blood Institute (NHLBI); membership of the steering committee for Afferent/Merck, Boehringer Ingelheim Ltd, Biogen, Bristol Myers Squibb, Chiesi, DevPro, GSK, Nitto, Patara/Respivant, Roche and Vicore; consulting (not receiving fees) for AstraZeneca, Boehringer Ingelheim, Excalibur, GSK, Hoffmann-La Roche, Lung Therapeutics, RS BioTherapeutics and Two XR; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer Ingelheim; support from Boehringer Ingelheim and Chiesi; participation on a data safety monitoring board or advisory board for Endeavor BioMedicines; and receipt of medical writing support from Boehringer Ingelheim and Hoffmann-La Roche. H. Parfrey declares grant payment to her institution from Boehringer Ingelheim Ltd; declares consulting fees from Boehringer Ingelheim Ltd, Roche Limited, Trevi Therapeutics and Pliant Therapeutics; declares speaker fees from Boehringer Ingelheim Ltd; and is a member of the Treating people with Idiopathic Pulmonary fibrosis with the Addition of Lansoprazole (TIPAL) trial management group, a trustee for Action for Pulmonary Fibrosis and a member of the scientific advisory board for the European Pulmonary Fibrosis Federation. I. Noth declares funding from NIH (UG3HL145266) to his institution; grant funding to his institution from Veracyte; and consulting fees from Boehringer Ingelheim and Sanofi. I.P. Hall declares funding from the Wellcome Trust and the National Institute for Health and Care Research (NIHR), and is vice chair of the Trustees for Asthma+Lung UK. J.M. Oldham declares funding from NIH (R01HL169166 & K23HL138190); consulting fees from Boehringer Ingelheim, Lupin Pharmaceuticals, AmMax Bio, Roche and Veracyte; a patent for TOLLIP TT genotype for N-acetylcysteine (NAC) use in IPF; participation on a data safety monitoring board or advisory board for Endeavor BioMedicines, Novartis and Genentech; is an associate editor for Chest; and is on the Program Committee for the American Thoracic Society and the editorial board for American Journal of Respiratory and Critical Care Medicine. L.V. Wain declares funding from UK Research and Innovation (MR/V00235X/1) and GSK/Asthma+Lung UK (Professorship (C17-1)) to complete this work; funding from Orion Pharma, GSK, Genentech, AstraZeneca, Nordic Bioscience and Sysmex (OGT); consulting fees from Galapagos, Boehringer Ingelheim and GSK; support for attending meetings and/or travel from Genentech; participation on an advisory board for Galapagos; and leadership or fiduciary roles as associate editor for the European Respiratory Journal and Medical Research Council board member and deputy chair. M.K.B. Whyte declares funding from NIH (K23HL146942); consulting fees from Genentech, Inogen, Medscape, AbbVie, patientMpower and Boehringer Ingelheim; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer Ingelheim. M. Neighbors is a full-time employee of Genentech/Roche with stock and stock options in Roche. M.D. Tobin declares a research collaboration with Orion Pharma unrelated to this manuscript, and a Respiratory Drug Delivery 2023 speaker fee, unrelated to the manuscript. M.R. Hill declares funding from the Wellcome Trust to University College London. N. Kaminski declares grant funding from NIH; grant funding to his institution from Bristol Myers Squibb, Boehringer Ingelheim and the Three Lakes Foundation; consultancy fees from Biogen Idec, Boehringer Ingelheim, Third Rock, Pliant, Samumed (now known as Biosplice Therapeutics) , NuMedii, Theravance, Three Lakes Partners, AstraZeneca, RohBar, Veracyte, Augmanity, CSL Behring, Thyron, Gilead, Galapagos, Chiesi, Arrowhead, Sofinnova, GSK and Merck; patents for new therapies for IPF (Biotech), new therapies for acute respiratory distress syndrome (Biotech) and new biomarkers in IPF (Biotech); equity in Pliant and Thyron; and serving as the scientific founder of Thyron. P.L. Molyneaux declares grant funding to his institution from AstraZeneca; consultancy fees from Hoffman-La Roche, Boehringer Ingelheim, AstraZeneca, Trevi and Qureight; speaker fees from Boehringer Ingelheim and Hoffman-La Roche; and appears on the editorial board for ERJ Open Research. R.G. Jenkins declares funding from UK Research and Innovation (MR/V00235X/1); that their institute received funding from AstraZeneca, Biogen, Galecto, GSK, Nordic Biosciences, Redx and Pliant; consulting fees from AstraZeneca, Brainomix, Bristol Myers Squibb, Chiesi, CohBar, Daewoong, GSK, Veracyte, Resolution Therapeutics and Pliant; payment for lectures and presentations from Boehringer Ingelheim, Chiesi, Roche, patientMPower, AstraZeneca; payment for expert testimony from Pinsent Masons LLP; participation on a data safety monitoring board or advisory board for Boehringer Ingelheim, Galapagos and Vicore; a leadership or fiduciary role for NuMedii; and that they are the president of Action for Pulmonary Fibrosis. S.P. Hart declares grant funding to his institution from Boehringer Ingelheim; consulting fees from Trevi Therapeutics; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Chiesi and Trevi Therapeutics; support for attending meetings and/or travel from Chiesi; participation on a data safety monitoring board or advisory board for Trevi Therapeutics; and that he was chair for the British Thoracic Society (BTS) Standards of Care Committee (from November 2019 until November 2022), a trustee for Action for Pulmonary Fibrosis and on the editorial board for ERJ Open Research. T.E. Fingerlin declares grant fees from NIH paid to their institution, consulting fees from Eleven P15 and a patent for methods for predicting the risk of interstitial pneumonia. T.M. Maher declares consulting fees from Boehringer Ingelheim, Roche/Genentech, AstraZeneca, Bayer, Blade Therapeutics, Bristol Myers Squibb, CSL Behring, Galapagos, Galecto, GSK, IQVIA, Pfizer, Pliant, Respivant, Sanofi, Theravance, Trevi, Veracyte and Vicore; participation on a data safety monitoring board or advisory board for FibroGen, Blade Therapeutics and NeRRe. X.R. Sheng is a full-time employee of Genentech/Roche with stock and stock options in Roche. All other authors have nothing to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
