Vaccine Induced Seroreactivity Following Administration of ALVAC-HIV/AIDSVAX®B/E Identified by Common Anti-HIV Test Kits and Algorithms in Thailand
- PMID: 41031105
- PMCID: PMC12477840
- DOI: 10.1093/ofid/ofaf578
Vaccine Induced Seroreactivity Following Administration of ALVAC-HIV/AIDSVAX®B/E Identified by Common Anti-HIV Test Kits and Algorithms in Thailand
Abstract
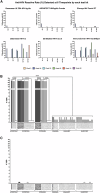
Vaccine-induced seroreactivity (VISR) was evaluated in RV306 and was shown to vary markedly (0-32.5%) among 6 HIV diagnostic tests and 84 algorithms. Our data show that selecting the SD Bioline HIV-1/2 assay and algorithms which exclude the ImmunoComb®II Bispot and Alere™ Determine HIV-1/2 assays would almost eliminate VISR in RV306.
Keywords: HIV-1; VISP; VISR; vaccine-induced seroreactivity.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2025.
Conflict of interest statement
Potential conflicts of interest. F. S. is an employee of Global Solutions for Infectious Diseases. J. T. and S. P. are employees of Sanofi Pasteur. All other authors declare no conflicts of interest.
Figures
References
-
- Quirk EK, Mogg R, Brown DD, et al. HIV seroconversion without infection after receipt of adenovirus-vectored HIV type 1 vaccine. Clin Infect Dis 2008; 47:1593–9. - PubMed
-
- Silbermann B, Tod M, Desaint C, et al. Short communication: long-term persistence of vaccine-induced HIV seropositivity among healthy volunteers. AIDS Res Hum Retroviruses 2008; 24:1445–8. - PubMed
-
- Aboud SMP, Joachim A, Bakari M, et al. poster P14.06. Persistence of Vaccine-Induced Antibodies Following HIV-1. DNA Prime MVA Boost Vaccination Among Healthy. Tanzanian Volunteers. AIDS Vaccine 2011; Global HIV Vaccine Enterprise, Bangkok, Thailand. 2011.
LinkOut - more resources
Full Text Sources

