Realization of differential release of minocycline hydrochloride from electrosprayed polymeric or biomacromolecular microparticles for the repair of traumatic brain injury
- PMID: 41031191
- PMCID: PMC12478139
- DOI: 10.1016/j.mtbio.2025.102309
Realization of differential release of minocycline hydrochloride from electrosprayed polymeric or biomacromolecular microparticles for the repair of traumatic brain injury
Abstract
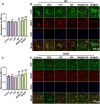
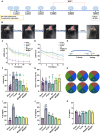
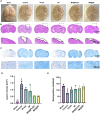
Traumatic brain injury (TBI) occurs when an external force impacts the brain and can result in various serious consequences. Currently, there are no clinical therapies to satisfy the different pathophysiological stages of TBI, realizing a combination of anti-inflammatories in the acute stage and polarization of M2 phenotype microglia. Herein, poly(lactic-co-glycolic acid) (PLGA) and silk fibroin (SF) were selected as shell layers, respectively, to fabricate minocycline hydrochloride (MH)-loaded core-shell microparticles through coaxial electrospray. MH@SF and MH@PLGA microparticles exhibited a differential release profile to promote nerve repair and regeneration after TBI. MH@SF achieved a fast release in 7 days to suppress neuroinflammatory response, while the sustained release of MH@PLGA up to 25 days allowed for modulating polarization of M2 phenotype microglia. The obtained microparticles promoted cell viability and neurite growth of primary neurons and SH-SY5Y cells by establishing neurite transection (NT) and oxygen-glucose deprivation (OGD) models to simulate primary and secondary injury after TBI. In vivo experiments further proved that MH@SF and MH@PLGA microparticles alleviate the neuroinflammation microenvironment and enhance motor, learning, and memory functions of mice after TBI. In summary, this work proposed a promising strategy to regulate the neuroimmune microenvironment through matching different pathophysiological stages of TBI, demonstrating potential for clinical translation to treat TBI.
Keywords: Differential release; Electrosprayed microparticle; Immune microenvironment; Neuroprotection; Traumatic brain injury.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Pavlovic D., Pekic S., Stojanovic M., Popovic V. Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary. 2019;22(3):270–282. - PubMed
-
- Liu X., Wu C., Zhang Y., Li G., Chen S., Chen Z., Liu P., Wu K., Wu X., Zhou T., Zeng M., Qiao Z., Xiao J., Ding J., Wei D., Sun J., Xu J., Zhou L., Fan H. Viscoelastic cues to induce stem cell migration and neuronal differentiation in cell-free hydrogel-assisted TBI recovery. Chem. Eng. J. 2024;492
-
- Liu X., Wu C., Zhang Y., Chen S., Ding J., Chen Z., Wu K., Wu X., Zhou T., Zeng M., Wei D., Sun J., Fan H., Zhou L. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023;306 - PubMed
-
- Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

