Modified Unipolar Return Pulsed Field Ablation in Ventricular Myocardium
- PMID: 41031398
- PMCID: PMC12529984
- DOI: 10.1161/CIRCEP.125.014006
Modified Unipolar Return Pulsed Field Ablation in Ventricular Myocardium
Abstract
Background: Various pulsed field ablation (PFA) parameters have been proposed to improve lesion depth. This study evaluated a modified unipolar return PFA system to create deep lesions in healthy and infarcted ventricular myocardia.
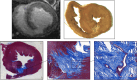
Methods: Numerical modeling was used to compare a modified unipolar return PFA system configuration with a conventional unipolar return (skin patch). We then performed ablation in 14 swine (5 with chronic myocardial infarction and 9 healthy). PFA lesions were created in the left ventricle using a focal catheter (4-mm tip) with a return electrode positioned in the inferior vena cava (biphasic, microsecond pulses of 1300 and 1500 V, 1-16 trains). Electroanatomical mapping guided ablation and lesion localization on magnetic resonance imaging were performed 48 hours post-ablation in the infarcted group and at 1 day, 7 days, and 6 weeks post-ablation in the healthy group.
Results: Numerical modeling demonstrated that the modified unipolar return PFA system produced deeper lesions with reduced variability compared with the skin patch. In healthy pigs (n=35 lesions), depths of 6.8±1.8 mm and widths of 11.5±4.7 mm were achieved with 8 pulse trains. Depths of 8.2±2.8 mm and widths of 14.0±4.7 mm were achieved with 16 trains. The maximum lesion depths were 8.8 and 11.6 mm for 8 and 16 trains, respectively. In the infarcted cohort (n=22 lesions), all lesions applied to scar tissue penetrated through fibrotic regions, with epicardial involvement observed in 57% of lesions.
Conclusions: The modified unipolar return PFA system effectively creates large lesions and can achieve transmurality in healthy and infarcted animals. Compared with conventional unipolar, it may offer greater lesion depth, width, and consistency.
Keywords: atrial fibrillation; catheter ablation; heart ventricles; pulmonary veins; tachycardia, ventricular.
Conflict of interest statement
Dr Verma reports clinical trials and advisory with Biosense Webster, Medtronic, Abbott, Medlumics, Adagio Medical, and Volta Medical. Dr Miklavčič reports advisory and co-patents with Medtronic. N. Coulombe and Drs Mattison and Sigg are employees of Medtronic. The other authors report no conflicts.
Figures





References
-
- Verma A, Boersma L, Haines DE, Natale A, Marchlinski FE, Sanders P, Calkins H, Packer DL, Hummel J, Onal B, et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrhythm Electrophysiol. 2022;15:e010168. doi: 10.1161/CIRCEP.121.010168 - PMC - PubMed
-
- Turagam MK, Neuzil P, Schmidt B, Reichlin T, Neven K, Metzner A, Hansen J, Blaauw Y, Maury P, Arentz T, et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF registry. Circulation. 2023;148:35–46. doi: 10.1161/CIRCULATIONAHA.123.064959 - PubMed
-
- Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, Mountantonakis SE, Gibson DN, Harding JD, Ellis CR, et al. ; ADVENT Investigators. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. 2023;389:1660–1671. doi: 10.1056/NEJMoa2307291 - PubMed
-
- Reichlin T, Kueffer T, Badertscher P, Jüni P, Knecht S, Thalmann G, Kozhuharov N, Krisai P, Jufer C, Maurhofer J, et al. ; SINGLE SHOT CHAMPION Investigators. Pulsed field or cryoballoon ablation for paroxysmal atrial fibrillation. N Engl J Med. 2025;392:1497–1507. doi: 10.1056/NEJMoa2502280 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

