Macrophage DNases Limit Neutrophil Extracellular Trap-Mediated Defective Efferocytosis in Atherosclerosis
- PMID: 41031413
- PMCID: PMC12542999
- DOI: 10.1161/CIRCRESAHA.125.326353
Macrophage DNases Limit Neutrophil Extracellular Trap-Mediated Defective Efferocytosis in Atherosclerosis
Abstract
Background: Neutrophil extracellular traps (NETs) contribute to atherosclerosis progression and are linked to adverse clinical outcomes such as myocardial infarction and stroke. Although the triggers of NET formation in plaques are known, the mechanisms governing DNase-mediated NET clearance and how these are disrupted during atherosclerosis remain unclear. Moreover, the consequences of impaired NET clearance on disease progression are not known.
Methods: Low-density lipoprotein receptor knockout (Ldlr-/-) mice with hematopoietic cell-specific deletion of DNase1 and DNase1L3 were fed a Western-type diet for 16 weeks to examine the impact of loss of DNase activity and the subsequent NET accumulation on advanced atherosclerosis. The effect of NETs on macrophage efferocytosis was examined in vitro and in the mouse peritoneal cavity and atherosclerotic plaque in vivo. To identify the signaling pathway impairing the NET-induced DNase response, in vitro assays were performed using selective endoplasmic reticulum stress pathway inhibitors, and the findings were validated in murine and human atherosclerotic tissues.
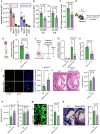
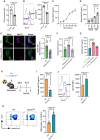
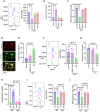
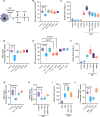
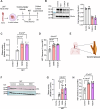
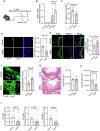
Results: Lack of DNase secretion by macrophages led to accumulation of NETs in local tissues, including atherosclerotic plaques. Persisting NETs in turn promoted cleavage of the efferocytosis receptor MerTK (c-mer proto-oncogene tyrosine kinase), resulting in defective macrophage efferocytosis and increased atherosclerotic plaque necrosis. In vitro screening identified endoplasmic reticulum stress-induced activation of the PERK (protein kinase R-like endoplasmic reticulum kinase)-ATF (activating transcription factor) 4 signaling axis in atherogenic macrophages as a key driver of impaired DNase secretion, leading to delayed NET clearance and their pathological persistence. Treatment of human atherosclerotic plaques and Ldlr-/- mice with integrated stress response inhibitor, a selective PERK inhibitor, restored vascular DNase secretion and facilitated NET clearance.
Conclusions: Macrophages play a key role in clearing NETs from tissues. Endoplasmic reticulum stress suppresses macrophage DNase secretion, leading to NET accumulation in atherosclerotic plaques, which triggers efferocytosis impairment and plaque progression. Targeting the PERK-ATF4 axis to restore DNase release and NET clearance represents a promising therapeutic strategy to promote plaque stabilization.
Keywords: atherosclerosis; efferocytosis; endoplasmic reticulum stress; extracellular traps; macrophages; neutrophils.
Conflict of interest statement
None.
Figures

Comment in
-
Ensnarement of Stressed Macrophages by NETs in Atherosclerosis.Circ Res. 2025 Oct 24;137(10):1276-1278. doi: 10.1161/CIRCRESAHA.125.327423. Epub 2025 Oct 23. Circ Res. 2025. PMID: 41129610 No abstract available.
References
-
- Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ, Boehme AK, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149:e347–e913. doi: 10.1161/CIR.0000000000001209 - PMC - PubMed
-
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005 - PubMed
-
- Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37:1720–1722. doi: 10.1093/eurheartj/ehw024 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

