Pharmacological targeting of the IL-17/neutrophil axis attenuates calcific deposits in rat models of calciphylaxis
- PMID: 41031886
- PMCID: PMC12483568
- DOI: 10.1172/JCI190369
Pharmacological targeting of the IL-17/neutrophil axis attenuates calcific deposits in rat models of calciphylaxis
Abstract
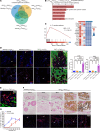
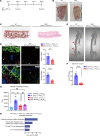
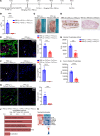
Calciphylaxis is a rare but life-threatening disorder characterized by ectopic calcification affecting the subcutaneous tissues and blood vessels of the skin. Survival rates are less than a year after diagnosis, and yet despite the severity of the condition, the pathobiology of calciphylaxis is ill understood. Here, we created animal models of calciphylaxis that recapitulated many characteristics of the human phenotype. We demonstrate that cutaneous calcification is preceded by inflammatory cell infiltration. We show that increased local skin inflammation, regardless of the inciting cause, in the presence of hypercalcemia and hyperphosphatemia contributes to cutaneous ectopic calcification. Genetically modified rodents lacking immune activation of T and B cells or NK cells are resistant to developing cutaneous calcification. Consistent with this, administration of the immunosuppressive cyclophosphamide reduced calcific deposits, as did T cell suppression with cyclosporine. We demonstrate that IL-17 is upregulated in calcific skin and neutrophils are the predominant cell type expressing IL-17 and tissue-nonspecific alkaline phosphatase (TNAP) that are necessary for ectopic calcification. Targeting IL-17 with a monoclonal antibody or using a myeloperoxidase inhibitor to blunt neutrophil activation notably attenuated calcific deposits in vivo. Taken together, these observations provide fresh insight into the role of the immune system and the IL-17/neutrophil axis in mediating ectopic calcification in rodent models of calciphylaxis.
Keywords: Bone disease; Cell biology; Dermatology; Neutrophils; Skin.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

