PROTACs in cancer immunotherapy: a minireview
- PMID: 41032705
- PMCID: PMC12599256
- DOI: 10.1042/BST20253065
PROTACs in cancer immunotherapy: a minireview
Abstract
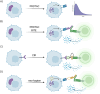
The discovery of immune checkpoint blockade as a therapeutic strategy to induce immunogenic cancer cell elimination has shown great success in the treatment of various cancers. However, limited response rates highlight the need for further development in this field. Promising new preclinical developments include the discoveries of proteolysis-targeting chimeras (PROTACs) to interfere with tumor immune escape signaling. Pharmacological induction of targeted protein degradation by these chimeras has shown advantages in inhibiting non-enzymatic protein functions and difficult to target protein-protein interactions. Furthermore, the induced degradation was shown to promote changes in the major histocompatibility complex I ligandome, which can be leveraged for an immune stimulus, increasing the cancer immune response. In this minireview, we highlight the research efforts ongoing towards employing PROTACs in immunotherapy for cancer treatment. Specifically, we outline how the unique mechanism of action can be leveraged to enhance the immune response or inhibit immune suppression.
Keywords: cancer; immune response; ubiquitin ligases; ubiquitin proteasome system; ubiquitin signaling.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

