The slit diaphragm in Drosophila exhibits a bilayered, fishnet architecture
- PMID: 41034222
- PMCID: PMC12489029
- DOI: 10.1038/s41467-025-64347-5
The slit diaphragm in Drosophila exhibits a bilayered, fishnet architecture
Abstract
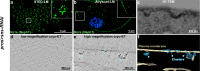
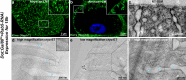
The kidney relies on the glomerulus to filter large volumes of blood plasma, with the slit diaphragm (SD) as a key structural component of the glomerular filtration barrier. Despite its central role, the molecular architecture of the SD has remained elusive for decades. Using cryo-electron tomography on focused ion beam-milled Drosophila nephrocytes, an invertebrate podocyte model, we show that the SD exhibits a bilayered fishnet architecture. In the cryo-electron tomography map, we observe criss-crossing strands spanning the extracellular space that can be populated with Sns and Kirre, the Drosophila orthologs of nephrin and Neph1, respectively. We show that sns silencing shortens the SD lines until disappearance, linking the fishnet architecture directly to Sns. After Rab5 silencing, which causes Sns mistrafficking and ectopic formation of the SD, the fishnet pattern also appears ectopically. Elucidating the molecular SD architecture establishes a crucial link between the SD organization and its (patho)physiology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- GRK 2566/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GRK 2566/1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GRK 2566/1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- CRC 1192/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GR3933/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- GR3933/1-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- TRR-422/Deutsche Forschungsgemeinschaft (German Research Foundation)
- FR 1653/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- HE 7456/7-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 431984000 - SFB 1453/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

