Real-world management strategies and clinical outcomes of metastatic HER2-positive breast cancer in Greece in the second-line setting and beyond (the togetHER study)
- PMID: 41034771
- PMCID: PMC12487351
- DOI: 10.1186/s12885-025-14791-9
Real-world management strategies and clinical outcomes of metastatic HER2-positive breast cancer in Greece in the second-line setting and beyond (the togetHER study)
Abstract
Background: In the realm of HER2-positive (HER2+) metastatic breast cancer (MBC), the advent of targeted therapies, notably trastuzumab and pertuzumab, represents a substantial advancement in enhancing patient outcomes. However, the intricacies of treatment escalate when patients encounter progression or resistance during first-line (1L) therapy. The togetHER study aimed to elucidate real-world insights into the profile, management strategies, and outcomes for HER2+ MBC patients initiating second-line treatment (2LT) in Greece, predating recent international guideline changes incorporating trastuzumab deruxtecan and tucatinib in the second line and beyond lines of treatments.
Methods: Data from adult female patients with HER2+ MBC who had initiated 2LT between 01-Jan-2015 and 31-Dec-2018 in 18 oncology departments across Greece were retrospectively abstracted through medical chart review.
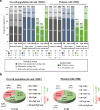
Results: The eligible population comprised 122 patients, 68.0% of whom presented with recurrent MBC. Among the latter, 26.5% were tested for HER2 both in early and metastatic settings with 27.3% of them changing HER2 status from negative to positive. Retesting of HER2 expression following 2LT initiation was recorded in 8 cases. At 2LT initiation, patients' median age was 57.0 years and 63.6% were hormone receptor-positive. The most common metastatic sites were bone (56.6%), lung (44.3%), liver (41.0%), and brain (29.5%). Anti-HER2 agent usage in 1L and 2L stood at 91.8% and 92.6%, respectively, with rates slightly diminishing in third (3L) (85.9%) and fourth line (4L) (82.4%). Endocrine therapy administration was generally low across treatment lines (12.3%, 9.9%, and 5.9%, in 2L, 3L, and 4L respectively), while chemotherapy use increased from 30.3% in 2L to 47.9% and 47.1% to 3L and 4L, correspondingly. Median progression-free survival (PFS) for 2L, 3L, and 4L was 7.7 [95% confidence interval (CI) 6.0-14.4], 6.4 (95% CI 5.4-7.1), and 5.6 (95% CI 2.6-9.0) months, respectively, while median overall survival was 25.0 (95% CI 20.5-34.4) months.
Conclusions: Although the 2LT pattern in Greece generally aligned with guidelines, persistently poor treatment outcomes underscore a significant unmet medical need for these patients.
Keywords: Anti-HER2; Metastatic breast cancer; OS; PFS; Real-world; Treatment patterns.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The present study was designed, conducted and reported in accordance with the ethical principles laid down in the Declaration of Helsinki, the Guidelines for Good Pharmacoepidemiology Practice of the International Society for Pharmacoepidemiology, the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines where applicable, the EU General Data Protection Regulation, and the local rules and regulations. In compliance with the latter, the final protocol of this Observational Study, including the final version of the Subject ICF, along with any amendments have been approved in writing by the Institutional Review Board of each participating hospital. National Ethics Committee (NEC) approval was waived based on local legislation. Informed consent was obtained from all individual participants included in the study except for deceased patients for whom a waiver of consent has been granted by the Institutional Review Board of each study site. Consent for publication: Not applicable. Competing interests: Ippokratis Korantzis, Anna Koumarianou, Vassiliki Rapti, Adamantia Nikolaidi, Vasileios Barbounis, Athina Christopoulou, Dimitrios Mavroudis, Vassilios Georgoulias, Georgios Kesisis, and Alexandros Ardavanis have no relevant financial or non-financial interests to disclose; Eleni Timotheadou has received research funding and honoraria from Roche, AstraZeneca, Amgen, Genesis, Pfizer, GSK, MSD, Novartis; Christos Christodoulou declared financial or non-financial interest with Amgen, AstraZeneca, BMS, Genesis, Gilead, Lilly, Merck, MSD, Novartis, Pfizer, Roche; Dimitrios Tryfonopoulos has received honoraria from Roche, AstraZeneca, Genesis-pharma; Sofia Karageorgopoulou has received honoraria for satellite lectures/advisory boards and research compensation and/or conference registration fees and travel grants from Amgen, AstraZeneca, Gilead Sciences, Genesis Pharma, GlaxoSmithKline, MSD, Novartis, Pfizer, Roche, Teva; Ioannis Boukovinas has received honoraria from Roche, MSD, Bristol-Myers Squibb, Pfizer, Novartis, Merck, AstraZeneca, LEO Pharma, Servier, consulting or advisory role fees from Roche, Sanofi, AstraZeneca, Bristol-Myers Squibb, LEO Pharma, MSD, Novartis, Ipsen, Genesis Pharma, research funding from Roche, Novartis, Bristol-Myers Squibb, MSD, Regeneron, Boehringer Ingelheim, Lilly, Pfizer, and travel, accommodations, expenses support from MSD, Roche, Pfizer, Bristol-Myers Squibb; Flora Zagouri has received honoraria for lectures and has served in an advisory role for AstraZeneca, Daiichi, Eli- Lilly, Merck, Novartis, MSD, Pfizer, Genesis- Pharma and Roche; Konstantinos Papazisis has received honoraria and consultancy fees from MSD, Gilead, AstraZeneca, Novartis, Genesis, IPSEN, Eli Lilly, Roche and GSK, research funding from Roche, MSD, Novartis, Daiichi Sankyo, Eli Lilly, Nektar, AstraZeneca, BMS, Sanofi, Boehringer and EISAI; Evangelia Razis has received travel grants from BMS, Roche, Pfizer, Karyo, Gilead Sciences and Genesis Pharma. Furthermore, Dr Razis has received honoraria from Servier pharmaceuticals and research funding from Tesaro, IQvia, AstraZeneca, Exelixis, PPD Global and MSD. Dr Razis has served in consulting/advisory role for AstraZeneca; Emmanuel I Papadopoulos and Rozalia Michalea are employees of AstraZeneca Greece. The Study was designed collaboratively by the Sponsor and Investigators. The sponsor had no involvement in the collection, analysis, and interpretation of data. A Medical writing vendor was used and paid by the sponsor to compose the first draft of the manuscript which was reviewed and approved by all authors who unanimously made the decision to submit the manuscript for publication.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. lyon, france: international agency for research on cancer. Available from: https://gco.iarc.who.int/today. Accessed 19 March 2024.
-
- National Cancer Institute (NCI). Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer. Available from: https://seer.cancer.gov/statfacts/html/breast.html. Accessed 22 February 2024.
-
- Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

