Discovery of therapeutic AGC2 modulators by combining docking, binding, and vesicle-based transport assays
- PMID: 41034876
- PMCID: PMC12486968
- DOI: 10.1186/s12967-025-06961-x
Discovery of therapeutic AGC2 modulators by combining docking, binding, and vesicle-based transport assays
Abstract
Backgroud: The mitochondrial Aspartate/Glutamate Carrier 2 (AGC2), encoded by the SLC25A13 gene, plays a critical role in cellular metabolism and redox balance through the malate/aspartate shuttle. Dysregulation of AGC2 is implicated in rare genetic diseases and tumorigenesis, making it a promising therapeutic target.
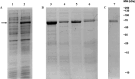
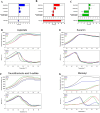
Methods: In this study, we developed the first integrative platform for the discovery and validation of high-affinity AGC2 modulators, combining in silico screening with biophysical and functional assays. Docking-based virtual screening of chemical libraries was employed to identify candidate inhibitors. Their binding and inhibitory activity were validated via a combination of thermal shift assays and isothermal titration calorimetry (ITC) performed on n-dodecyl-β-D-maltoside (DDM)-based vesicles reconstituted with AGC2, alongside functional transport assays using AGC2-containing proteoliposomes.
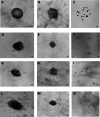
Results: We identified two previously unreported AGC2 inhibitors, suramin and taurolithocholic acid 3-sulfate. Remarkably, we report the first successful application of ITC to AGC2, overcoming major experimental challenges associated with ITC assays on the SLC25A family members, and achieving greater stability and reproducibility compared to similar assays performed on other family members, such as the ADP/ATP carrier and uncoupling proteins. Additionally, we present the first transmission electron microscopy (TEM) characterization of proteoliposomes and DDM-based vesicles reconstituted with AGC2, providing direct structural insights into the systems used for biophysical analysis.
Conclusions: This study establishes a reproducible, and scalable workflow that bridges high-throughput ligand identification with high-resolution kinetic characterization for targeting mitochondrial carriers.
Keywords: Aralar, SLC25A12 (Aspartate/glutamate carrier paralog 1); Aspartate/glutamate carrier (AGC); Citrin, SLC25A13 (Aspartate/glutamate carrier paralog 2); Isothermal titration calorimetry; Mitochondrial carrier; Mitochondrial carrier inhibitors; Mitochondrial transporter; SLC25A family; Thermostability shift assay; Transmission electron microscopy; Virtual screening.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no conflicts of interest.
Figures







References
-
- Palmieri F, Pierri CL. Mitochondrial metabolite transport. Essays Biochem. 2010;47:37–52. - PubMed
-
- Saraste M, Walker JE. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett. 1982;144:250–4. - PubMed
-
- Palmieri F, Pierri CL. Structure and function of mitochondrial carriers—role of the transmembrane helix P and G residues in the gating and transport mechanism. FEBS Lett. 2010;584:1931–9. - PubMed
-
- Giangregorio N, Pierri CL, Tonazzi A, Incampo G, Tragni V, De Grassi A, et al. Proline/Glycine residues of the PG-levels guide conformational changes along the transport cycle in the mitochondrial carnitine/acylcarnitine carrier (SLC25A20). Int J Biol Macromol. 2022;221:1453–65. 10.1016/j.ijbiomac.2022.09.135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

