High-throughput drug screening in advanced pre-clinical 3D melanoma models identifies potential first-line therapies for NRAS-mutated melanoma
- PMID: 41035005
- PMCID: PMC12487241
- DOI: 10.1186/s13046-025-03539-9
High-throughput drug screening in advanced pre-clinical 3D melanoma models identifies potential first-line therapies for NRAS-mutated melanoma
Abstract
Background: Despite significant advances in targeted (BRAFi + MEKi) and immune (anti-PD1/PD-L1, anti-CTLA4, and anti-LAG3) therapies, treatment options for NRASmut melanoma remain limited. Currently, NRASmut patients rely on immune checkpoint inhibitors, classical chemotherapy, and off-label MEK inhibitors, with over 50% experiencing rapid disease progression. One of the key challenges in developing effective targeted therapies is the lack of preclinical models that accurately recapitulate the tumor microenvironment (TME) and the intrinsic resistance of melanoma cells bearing NRAS mutations.
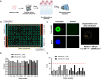
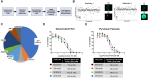
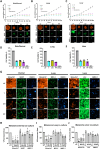
Methods: To address this, we performed high-throughput screening (HTS) of over 1,300 compounds in 3D NRASmut melanoma spheroids. A multi-step analysis was performed to identify hits, which were further tested by performing drug-response curve (DRC) analysis. Most promising compounds were further validated using mono- and co-culture 3D in vitro models that mimic three main metastatic sites in melanoma, such as skin/dermal, lung, and liver, utilizing spheroid and hydrogel systems. Ultimately, validation was conducted using zebrafish xenograft models to enable a more refined and accurate assessment of drug response.
Results: High-throughput drug screening of NRASmut melanoma spheroids identified 17 candidate compounds, which were subsequently validated through DRC analyses. Among the most promising drugs, Daunorubicin HCl (DH) and Pyrvinium Pamoate (PP) were selected for further investigation, demonstrating potent anti-melanoma activity in advanced 3D co-culture systems and zebrafish xenograft models. Notably, PP demonstrated higher cytotoxicity compared to Trametinib, the off-label MEK inhibitor, with an inhibitory effect on AKT and invasive behavior in the patient-derived metastatic melanoma cell lines. Additionally, combinatorial treatment with Trametinib resulted in additive effects on cell proliferation and viability. Importantly, both compounds showed similar efficacy in NRASmut and BRAFwt/NRASwt melanoma cell lines that were resistant to Trametinib (MEK inhibitor).
Conclusions: Using advanced 3D melanoma models that incorporate key TME elements and zebrafish xenograft models, this study highlights the potential of Daunorubicin HCl and Pyrvinium Pamoate as novel first-line therapies for NRASmut melanoma, with a noteworthy effect also on MEKi-resistant cells. These findings support drug repurposing strategies and underscore the importance of physiologically relevant preclinical models in identifying effective therapies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All patient-derived melanoma cell lines derived from metastatic melanoma tumors (fresh or slow frozen) were obtained from the repository of Prof. Mitchell Levesque of the Department of Dermatology, University Hospital Zurich, Switzerland. The repository was established for the approved clinical study: BASEC: 2018–02050, 2018–02052, 2019 − 01326. The Zebrafish Facility at the University of Padova holds the authorization 407/2015-PR (OPBA), and the Zebrafish Core Facility at the Luxembourg Centre for Systems Biomedicine at the University of Luxembourg is registered as an authorized breeder, supplier, and user of zebrafish with Grand-Ducal Decree of 26 January 2023. All practices involving zebrafish complied with the European Legislation for the Protection of Animals used for Scientific Purposes (Directive 2010/63/EU) and following the principles of the 3Rs. Consent for publication: All the authors read and approved the final manuscript. Competing interests: The authors declare no competing interests.
Figures



References
-
- Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous melanoma. Lancet. 2023;402(10400):485–502. - PubMed
-
- Shain AH, Bastian BC. From melanocytes to melanomas. Nat Reviews Cancer Nat Publishing Group. 2016;16:345–58. - PubMed
-
- Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB et al. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–96. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0092867415006340 - PMC - PubMed
-
- Boutros A, Croce E, Ferrari M, Gili R, Massaro G, Marconcini R et al. The treatment of advanced melanoma: Current approaches and new challenges. Crit Rev Oncol Hematol. 2024;196:104276. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1040842824000192 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

